Abstract
The five-year survival rate for patients with malignant glioma is less than 10 %. Despite aggressive chemo/radiotherapy these tumors have remained resistant to almost every interventional strategy evaluated in patients. Resistance to these agents is attributed to extrinsic mechanisms such as the tumor microenvironment, poor drug penetration, and tumoral heterogeneity. In addition, genetic and molecular examination of these tumors has revealed defective apoptotic regulation, enhanced pro-survival autophagy signaling, and a propensity for necrosis that aids in the adaptation to environmental stress and resistance to treatment. The combination of extrinsic and intrinsic hallmarks in glioma contributes to the multifaceted resistance to traditional anti-tumor agents. Here we describe the biology of the disease relevant to therapeutic resistance, with a specific focus on molecular deregulation of cell death pathways. Emerging studies investigating the targeting of these pathways including BH3 mimetics and autophagy inhibitors that are being evaluated in both the preclinical and clinical settings are discussed. This review highlights the pathways exploited by glioblastoma cells that drive their hallmark pro-survival predisposition and makes therapy development such a challenge.



Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Jiang YG, Peng Y, Koussougbo KS (2011) Necroptosis: a novel therapeutic target for glioblastoma. Med Hypotheses 76(3):350–352
Tait SW, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11(9):621–632
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR (2010) The BCL-2 family reunion. Mol Cell 37(3):299–310
Cartron PF, Loussouarn D, Campone M, Martin SA, Vallette FM (2012) Prognostic impact of the expression/phosphorylation of the BH3-only proteins of the BCL-2 family in glioblastoma multiforme. Cell Death Dis 3:e421
Strik H, Deininger M, Streffer J, Grote E, Wickboldt J, Dichgans J et al (1999) BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radio-chemotherapy. J Neurol Neurosurg Psychiatry 67(6):763–768
Qiu B, Wang Y, Tao J, Wang Y (2012) Expression and correlation of Bcl-2 with pathological grades in human glioma stem cells. Oncol Rep 28(1):155–160
Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ (1998) Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA 95(10):5724–5729
Zhu H, Cao X, Ali-Osman F, Keir S, Lo HW (2010) EGFR and EGFRvIII interact with PUMA to inhibit mitochondrial trans-localization of PUMA and PUMA-mediated apoptosis independent of EGFR kinase activity. Cancer Lett 294(1):101–110
Stegh AH, Kim H, Bachoo RM, Forloney KL, Zhang J, Schulze H et al (2007) Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev 21(1):98–111
Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A et al (2008) Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA 105(31):10703–10708
Stegh AH, Brennan C, Mahoney JA, Forloney KL, Jenq HT, Luciano JP et al (2010) Glioma oncoprotein Bcl2L12 inhibits the p53 tumor suppressor. Genes Dev 24(19):2194–2204
Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Bock BC et al (2008) Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene 27(52):6646–6656
Manero F, Gautier F, Gallenne T, Cauquil N, Gree D, Cartron PF et al (2006) The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res 66(5):2757–2764
Dodou K, Anderson RJ, Small DA, Groundwater PW (2005) Investigations on gossypol: past and present developments. Expert Opin Investig Drugs 14(11):1419–1434
Wagenknecht B, Glaser T, Naumann U, Kugler S, Isenmann S, Bahr M et al (1999) Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ 6(4):370–376
Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ et al (2002) Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol 20(4):1063–1068
Ziegler DS, Wright RD, Kesari S, Lemieux ME, Tran MA, Jain M et al (2008) Resistance of human glioblastoma multiforme cells to growth factor inhibitors is overcome by blockade of inhibitor of apoptosis proteins. J Clin Investig 118(9):3109–3122
Chakravarti A, Zhai GG, Zhang M, Malhotra R, Latham DE, Delaney MA et al (2004) Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene 23(45):7494–7506
Fulda S, Wick W, Weller M, Debatin KM (2002) Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med 8(8):808–815
Wagner L, Marschall V, Karl S, Cristofanon S, Zobel K, Deshayes K et al (2013) Smac mimetic sensitizes glioblastoma cells to Temozolomide-induced apoptosis in a RIP1- and NF-kappaB-dependent manner. Oncogene 32(8):988–997
Begg AC, Stewart FA, Vens C (2011) Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 11(4):239–253
Venur VA, Peereboom DM, Ahluwalia MS (2015) Current medical treatment of glioblastoma. Cancer Treat Res 163:103–115
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Kondo N, Takahashi A, Ono K, Ohnishi T (2010) DNA damage induced by alkylating agents and repair pathways. J Nucleic Acids 2010:543531
Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W et al (2010) MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6(1):39–51
Barazzuol L, Jena R, Burnet NG, Meira LB, Jeynes JC, Kirkby KJ et al (2013) Evaluation of poly (ADP-ribose) polymerase inhibitor ABT-888 combined with radiotherapy and temozolomide in glioblastoma. Radiat Oncol 8:65
Lin F, de Gooijer MC, Roig EM, Buil LC, Christner SM, Beumer JH et al (2014) ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin Cancer Res Off J Am Assoc Cancer Res 20(10):2703–2713
Tentori L, Leonetti C, Scarsella M, D’Amati G, Vergati M, Portarena I et al (2003) Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res Off J Am Assoc Cancer Res 9(14):5370–5379
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ et al (2007) ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res Off J Am Assoc Cancer Res 13(9):2728–2737
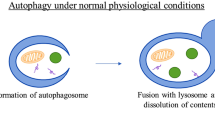
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42
Liu EY, Ryan KM (2012) Autophagy and cancer–issues we need to digest. J Cell Sci 125(Pt 10):2349–2358
White E, DiPaola RS (2009) The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 15(17):5308–5316
Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T et al (2010) Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal 3(147):ra81
Shen J, Zheng H, Ruan J, Fang W, Li A, Tian G et al (2013) Autophagy inhibition induces enhanced proapoptotic effects of ZD6474 in glioblastoma. Br J Cancer 109(1):164–171
Gammoh N, Lam D, Puente C, Ganley I, Marks PA, Jiang X (2012) Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death. Proc Natl Acad Sci USA 109(17):6561–6565
Li Y, Zhu H, Zeng X, Fan J, Qian X, Wang S et al (2013) Suppression of autophagy enhanced growth inhibition and apoptosis of interferon-beta in human glioma cells. Mol Neurobiol 47(3):1000–1010
Ge PF, Zhang JZ, Wang XF, Meng FK, Li WC, Luan YX et al (2009) Inhibition of autophagy induced by proteasome inhibition increases cell death in human SHG-44 glioma cells. Acta Pharmacol Sin 30(7):1046–1052
Chen Y, Meng D, Wang H, Sun R, Wang D, Wang S, et al (2014) VAMP8 facilitates cellular proliferation and temozolomide resistance in human glioma cells. Neuro Oncol 17(3):407–418
Cho HY, Wang W, Jhaveri N, Lee DJ, Sharma N, Dubeau L et al (2014) NEO212, temozolomide conjugated to perillyl alcohol, is a novel drug for effective treatment of a broad range of temozolomide-resistant gliomas. Mol Cancer Ther 13(8):2004–2017
Curry RC, Dahiya S, Alva Venur V, Raizer JJ, Ahluwalia MS (2015) Bevacizumab in high-grade gliomas: past, present, and future. Expert Rev Anticancer Ther 15(4):387–397
Gilbert MR, Sulman EP, Mehta MP (2014) Bevacizumab for newly diagnosed glioblastoma. New Engl J Med 370(21):2048–2049
Chinot OL, Wick W, Cloughesy T (2014) Bevacizumab for newly diagnosed glioblastoma. New Engl J Med 370(21):2049
Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS et al (2012) Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res 72(7):1773–1783
Golden EB, Cho HY, Jahanian A, Hofman FM, Louie SG, Schonthal AH et al (2014) Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg Focus 37(6):E12
Rosenfeld MR, Ye X, Supko JG, Desideri S, Grossman SA, Brem S et al (2014) A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy 10(8):1359–1368
Alonso MM, Jiang H, Yokoyama T, Xu J, Bekele NB, Lang FF et al (2008) Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther 16(3):487–493
Zong WX, Thompson CB (2006) Necrotic death as a cell fate. Genes Dev 20(1):1–15
Stegh AH, Chin L, Louis DN, DePinho RA (2008) What drives intense apoptosis resistance and propensity for necrosis in glioblastoma? A role for Bcl2L12 as a multifunctional cell death regulator. Cell Cycle 7(18):2833–2839
Flannery T, McQuaid S, McGoohan C, McConnell RS, McGregor G, Mirakhur M et al (2006) Cathepsin S expression: an independent prognostic factor in glioblastoma tumours—a pilot study. Int J Cancer J Int Du cancer 119(4):854–860
Keerthivasan S, Keerthivasan G, Mittal S, Chauhan SS (2007) Transcriptional upregulation of human cathepsin L by VEGF in glioblastoma cells. Gene 399(2):129–136
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45(6):487–498
Melo-Lima S, Celeste Lopes M, Mollinedo F (2014) Necroptosis is associated with low procaspase-8 and active RIPK1 and -3 in human glioma cells. Oncoscience 1(10):649–664
Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D (2014) Activation of the NLRP3 inflammasome by proteins that signal for necroptosis. Methods Enzymol 545:67–81
Reardon DA, Wucherpfennig KW, Freeman G, Wu CJ, Chiocca EA, Wen PY et al (2013) An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines 12(6):597–615
Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V et al (2012) NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48(14):2192–2202
Turk B, Turk V (2009) Lysosomes as “suicide bags” in cell death: myth or reality? J Biol Chem 284(33):21783–21787
Blomgran R, Zheng L, Stendahl O (2007) Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J Leukoc Biol 81(5):1213–1223
Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA (2013) Thymoquinone inhibits autophagy and induces cathepsin-mediated, caspase-independent cell death in glioblastoma cells. PLoS One 8(9):e72882
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27(50):6434–6451
Acknowledgments
The authors acknowledge Servier Medical Art Image Bank (http://www.servier.com/Powerpoint-image-bank) for the free image components used to create Figs. 1, 2, and 3 (http://creativecommons.org/licenses/by/3.0/legalcode).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wojton, J., Meisen, W.H. & Kaur, B. How to train glioma cells to die: molecular challenges in cell death. J Neurooncol 126, 377–384 (2016). https://doi.org/10.1007/s11060-015-1980-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1980-1