Abstract
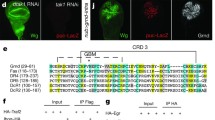
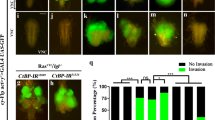
Platelet-derived growth factor receptor (PDGFR) signaling plays an important role in the biology of malignant gliomas. To investigate mechanisms modulating PDGFR signaling in gliomagenesis, we employed a Drosophila glioma model and genetic screen to identify genes interacting with Pvr, the fly homolog of PDGFRs. Glial expression of constitutively activated Pvr (λPvr) led to glial over migration and lethality at late larval stage. Among 3316 dsRNA strains crossed against the tester strain, 128 genes shifted lethality to pupal stage, including tetraspanin 2A (tsp2A). In a second step knockdown of all Drosophila tetraspanins was investigated. Of all tetraspanin dsRNA strains only knockdown of tsp2A partially rescued the Pvr-induced phenotype. Human CD9 (TSPAN29/MRP-1), a close homolog of tsp2A, was found to be expressed in glioma cell lines A172 and U343MG as well as in the majority of glioblastoma samples (16/22, 73 %). Furthermore, in situ proximity ligation assay revealed close association of CD9 with PDGFR α and β. In U343MG cells, knockdown of CD9 blocked PDGF-BB stimulated migration. In conclusion, modulation of PDGFR signaling by CD9 is evolutionarily conserved from Drosophila glia to human glioma and plays a role in glia migration.




Similar content being viewed by others
References
Cancer Genome Atlas research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. doi:10.1038/nature07385
Nazarenko I, Hede SM, He X, Hedren A, Thompson J, Lindstrom MS, Nister M (2012) PDGF and PDGF receptors in glioma. Upsala J Med Sci 117:99–112. doi:10.3109/03009734.2012.665097
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. doi:10.1016/j.ccr.2009.12.020
Ozawa T, Brennan CW, Wang L, Squatrito M, Sasayama T, Nakada M, Huse JT, Pedraza A, Utsuki S, Yasui Y, Tandon A, Fomchenko EI, Oka H, Levine RL, Fujii K, Ladanyi M, Holland EC (2010) PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev 24:2205–2218. doi:10.1101/gad.1972310
Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E (2009) Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One 4:e7752. doi:10.1371/journal.pone.0007752
Reardon DA, Desjardins A, Vredenburgh JJ, Herndon JE 2nd, Coan A, Gururangan S, Peters KB, McLendon R, Sathornsumetee S, Rich JN, Lipp ES, Janney D, Friedman HS (2012) Phase II study of Gleevec plus hydroxyurea in adults with progressive or recurrent low-grade glioma. Cancer 118:4759–4767. doi:10.1002/cncr.26541
Sjoblom T, Boureux A, Ronnstrand L, Heldin CH, Ghysdael J, Ostman A (1999) Characterization of the chronic myelomonocytic leukemia associated TEL-PDGF beta R fusion protein. Oncogene 18:7055–7062. doi:10.1038/sj.onc.1203190
Read RD, Cavenee WK, Furnari FB, Thomas JB (2009) A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet 5:e1000374. doi:10.1371/journal.pgen.1000374
Witte HT, Jeibmann A, Klambt C, Paulus W (2009) Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia 11:882–888
Piek E, Afrakhte M, Sampath K, van Zoelen EJ, Heldin CH, ten Dijke P (1999) Functional antagonism between activin and osteogenic protein-1 in human embryonal carcinoma cells. J Cell Physiol 180:141–149. doi:10.1002/(SICI)1097-4652(199908)180:2<141:AID-JCP1>3.0.CO;2-I
Piek E, Heldin CH, Ten Dijke P (1999) Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J 13:2105–2124
Hoch RV, Soriano P (2003) Roles of PDGF in animal development. Development 130:4769–4784. doi:10.1242/dev.00721
Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C (2008) Organization and function of the blood-brain barrier in Drosophila. J Neurosci 28:587–597. doi:10.1523/JNEUROSCI.4367-07.2008
Halter DA, Urban J, Rickert C, Ner SS, Ito K, Travers AA, Technau GM (1995) The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila melanogaster. Development 121:317–332
Lee BP, Jones BW (2005) Transcriptional regulation of the Drosophila glial gene repo. Mech Dev 122:849–862. doi:10.1016/j.mod.2005.01.002
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Mertsch S, Schurgers LJ, Weber K, Paulus W, Senner V (2009) Matrix gla protein (MGP): an overexpressed and migration-promoting mesenchymal component in glioblastoma. BMC Cancer 9:302. doi:10.1186/1471-2407-9-302
Koos B, Paulsson J, Jarvius M, Sanchez BC, Wrede B, Mertsch S, Jeibmann A, Kruse A, Peters O, Wolff JE, Galla HJ, Soderberg O, Paulus W, Ostman A, Hasselblatt M (2009) Platelet-derived growth factor receptor expression and activation in choroid plexus tumors. Am J Pathol 175:1631–1637. doi:10.2353/ajpath.2009.081022
Kamentsky L, Jones TR, Fraser A, Bray MA, Logan DJ, Madden KL, Ljosa V, Rueden C, Eliceiri KW, Carpenter AE (2011) Improved structure, function and compatibility for Cell Profiler: modular high-throughput image analysis software. Bioinformatics 27:1179–1180. doi:10.1093/bioinformatics/btr095
Kawashima M, Doh-ura K, Mekada E, Fukui M, Iwaki T (2002) CD9 expression in solid non-neuroepithelial tumors and infiltrative astrocytic tumors. J Histochem Cytochem 50:1195–1203
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283–1316
Podbielska M, Banik NL, Kurowska E, Hogan EL (2013) Myelin recovery in multiple sclerosis: the challenge of remyelination. Brain Sci 3:1282–1324. doi:10.3390/brainsci3031282
Kanakaraj P, Raj S, Khan SA, Bishayee S (1991) Ligand-induced interaction between alpha- and beta-type platelet-derived growth factor (PDGF) receptors: role of receptor heterodimers in kinase activation. Biochemistry 30:1761–1767
Tiwari-Woodruff SK, Buznikov AG, Vu TQ, Micevych PE, Chen K, Kornblum HI, Bronstein JM (2001) OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and β1 integrin and regulates proliferation and migration of oligodendrocytes. J Cell Biol 153:295–305
Mela A, Goldman JE (2009) The tetraspanin KAI1/CD82 is expressed by late-lineage oligodendrocyte precursors and may function to restrict precursor migration and promote oligodendrocyte differentiation and myelination. J Neurosci 29:11172–11181. doi:10.1523/JNEUROSCI.3075-09.2009
Anton ES, Hadjiargyrou M, Patterson PH, Matthew WD (1995) CD9 plays a role in Schwann cell migration in vitro. J Neurosci 15:584–595
del Valle Rodriguez A, Didiano D, Desplan C (2012) Power tools for gene expression and clonal analysis in Drosophila. Nat Methods 9:47–55. doi:10.1038/nmeth.1800
Brinck J, Heldin P (1999) Expression of recombinant hyaluronan synthase (HAS) isoforms in CHO cells reduces cell migration and cell surface CD44. Exp Cell Res 252:342–351. doi:10.1006/excr.1999.4645
Husmark J, Heldin NE, Nilsson M (1999) N-cadherin-mediated adhesion and aberrant catenin expression in anaplastic thyroid-carcinoma cell lines. Int J Cancer 83:692–699
Paulsson J, Lindh MB, Jarvius M, Puputti M, Nister M, Nupponen NN, Paulus W, Soderberg O, Dresemann G, von Deimling A, Joensuu H, Ostman A, Hasselblatt M (2011) Prognostic but not predictive role of platelet-derived growth factor receptors in patients with recurrent glioblastoma. Int J Cancer 128:1981–1988. doi:10.1002/ijc.25528
Heuchel R, Berg A, Tallquist M, Ahlen K, Reed RK, Rubin K, Claesson-Welsh L, Heldin CH, Soriano P (1999) Platelet-derived growth factor beta receptor regulates interstitial fluid homeostasis through phosphatidylinositol-3′ kinase signaling. Proc Natl Acad Sci USA 96:11410–11415
Shimizu A, O’Brien KP, Sjoblom T, Pietras K, Buchdunger E, Collins VP, Heldin CH, Dumanski JP, Ostman A (1999) The dermatofibrosarcoma protuberans-associated collagen type Iα1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res 59:3719–3723
Heldin NE, Bergstrom D, Hermansson A, Bergenstrahle A, Nakao A, Westermark B, ten Dijke P (1999) Lack of responsiveness to TGF-beta1 in a thyroid carcinoma cell line with functional type I and type II TGF-beta receptors and Smad proteins, suggests a novel mechanism for TGF-beta insensitivity in carcinoma cells. Mol Cell Endocrinol 153:79–90
Ikeyama S, Koyama M, Yamaoko M, Sasada R, Miyake M (1993) Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med 177:1231–1237
Takeda T, Hattori N, Tokuhara T, Nishimura Y, Yokoyama M, Miyake M (2007) Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res 67:1744–1749. doi:10.1158/0008-5472.CAN-06-3090
Saito Y, Tachibana I, Takeda Y, Yamane H, He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, Osaki T, Kawase I (2006) Absence of CD9 enhances adhesion-dependent morphologic differentiation, survival, and matrix metalloproteinase-2 production in small cell lung cancer cells. Cancer Res 66:9557–9565. doi:10.1158/0008-5472.CAN-06-1131
Murayama Y, Shinomura Y, Oritani K, Miyagawa J, Yoshida H, Nishida M, Katsube F, Shiraga M, Miyazaki T, Nakamoto T, Tsutsui S, Tamura S, Higashiyama S, Shimomura I, Hayashi N (2008) The tetraspanin CD9 modulates epidermal growth factor receptor signaling in cancer cells. J Cell Physiol 216:135–143. doi:10.1002/jcp.21384
Ronnstrand L, Siegbahn A, Rorsman C, Johnell M, Hansen K, Heldin CH (1999) Overactivation of phospholipase C-gamma1 renders platelet-derived growth factor beta-receptor-expressing cells independent of the phosphatidylinositol 3-kinase pathway for chemotaxis. J Biol Chem 274:22089–22094
Acknowledgments
Research was supported by DFG Grants (PA 328/7-1) and (Ko-4345/1-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeibmann, A., Halama, K., Witte, H.T. et al. Involvement of CD9 and PDGFR in migration is evolutionarily conserved from Drosophila glia to human glioma. J Neurooncol 124, 373–383 (2015). https://doi.org/10.1007/s11060-015-1864-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1864-4