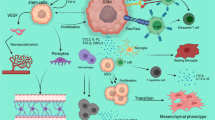
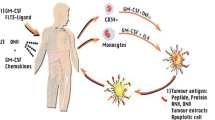
Abstract
In much of medical oncology, including neuro-oncology, there is great interest to evaluate the therapeutic potential of immune-based therapies including vaccines, adoptive T cell strategies and modulators of immune checkpoint regulators such as cytotoxic T lymphocyte antigen 4 and programmed death 1. Immune-based treatments exert an indirect anti-tumor effect by generating potent, tumor-targeting immune responses. Robust anti-tumor immune responses have been shown to achieve encouraging radiographic responses across the spectrum of applied immunotherapeutics which are felt to be indicative of a bona fide anti-tumor effect. Conversely, worsening of imaging findings, particularly early in the course of immunotherapy administration, can be challenging to interpret with growing evidence demonstrating that at least a subset of such patients ultimately will derive meaningful clinical benefit. The immune related response criteria were generated to provide guidance regarding the interpretation of such complex imaging findings, for general medical oncologists prescribing immunotherapeutics. An analogous effort that addresses challenges associated with imaging assessment and incorporates nuances associated with neuro-oncology patients is underway and is referred to as the immunotherapy response assessment in neuro-oncology criteria.


Similar content being viewed by others
References
Coley WB (1893) The treatment of malignant tumors by repeated inoculations of Erysipelas, with a report of ten original cases. Am J Med Sci 105:487–511
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909
Kantoff PW, Higano CS, Shore ND et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Reardon DA, Wucherpfennig KW, Freeman G et al (2013) An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines 12:597–615
Wen PY, Reardon DA, Phuphanich S, et al (2014) A randomized, double-blind, placebo-controlled phase 2 trial of dendritic cell (DC) vaccination with ICT-107 in newly diagnosed glioblastoma (GBM) patients, 2014 American Society of Clinical Oncology Annual Meeting, ASCO, Chicago, IL
Babu R, Adamson DC (2012) Rindopepimut: an evidence-based review of its therapeutic potential in the treatment of EGFRvIII-positive glioblastoma. Core Evid 7:93–103
Reardon DA, Li G, Recht L, et al (2013) ReACT: a phase II study of rindopepimut vaccine (CDX-110) plus bevacizumab in relapsed glioblastoma (GBM), 4th Quadrennial Meeting of the World Federation of Neuro-Oncology/18th Annual Meeting of the Society for Neuro-Oncology. Oxford Press, San Francisco, CA
Bloch O, Crane CA, Fuks Y et al (2014) Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro-Oncology 16:274–279
Maude SL, Frey N, Shaw PA et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507–1517
Grupp SA, Kalos M, Barrett D et al (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509–1518
Davila ML, Riviere I, Wang X et al (2014) Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6:224ra25
Brentjens RJ, Davila ML, Riviere I et al (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5:177ra38
Kochenderfer JN, Dudley ME, Feldman SA et al (2012) B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119:2709–2720
Gilham DE, Debets R, Pule M et al (2012) CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med 18:377–384
Ott PA, Hodi FS, Robert C (2013) CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 19:5300–5309
Sznol M, Chen L (2013) Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 19:1021–1034
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Robert C, Thomas L, Bondarenko I et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526
Prieto PA, Yang JC, Sherry RM et al (2012) CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 18:2039–2047
Robert C, Ribas A, Wolchok JD et al (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384:1109–1117
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Hamid O, Robert C, Daud A et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133
Shurin MR (2013) Dual role of immunomodulation by anticancer chemotherapy. Nat Med 19:20–22
Postow MA, Callahan MK, Barker CA et al (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366:925–931
Chinot OL, Macdonald DR, Abrey LE et al (2013) Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep 13:347
Brandsma D, Stalpers L, Taal W et al (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Radbruch A, Fladt J, Kickingereder P et al (2015) Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro-Oncol 17(1):151–159
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Little RF, Pluda JM, Wyvill KM et al (2006) Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 107:4650–4657
van Baren N, Bonnet MC, Dreno B et al (2005) Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol 23:9008–9021
Kruit WH, van Ojik HH, Brichard VG et al (2005) Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer 117:596–604
Di Giacomo AM, Danielli R, Guidoboni M et al (2009) Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 58:1297–1306
Brahmer JR, Tykodi SS, Chow LQ et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Topalian SL, Sznol M, McDermott DF et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030
Hodi FS, Lawrence D, Lezcano C et al (2014) Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2:632–642
Okada H, Kalinski P, Ueda R et al (2011) Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 29:330–336
Kirkwood JM, Lorigan P, Hersey P et al (2010) Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res 16:1042–1048
Hodi FS, Mihm MC, Soiffer RJ et al (2003) Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 100:4712–4717
Pollack IF, Jakacki RI, Butterfield LH et al (2014) Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol 32:2050–2058
Sampson JH, Heimberger AB, Archer GE et al (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729
Chiocca EA, Aguilar LK, Bell SD et al (2011) Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol 29:3611–3619
Hoos A, Eggermont AM, Janetzki S et al (2010) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102:1388–1397
Hoos A, Parmiani G, Hege K et al (2007) A clinical development paradigm for cancer vaccines and related biologics. J Immunother 30:1–15
Vrabec M, Van Cauter S, Himmelreich U et al (2011) MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology 53:721–731
Stenberg L, Englund E, Wirestam R et al (2006) Dynamic susceptibility contrast-enhanced perfusion magnetic resonance (MR) imaging combined with contrast-enhanced MR imaging in the follow-up of immunogene-treated glioblastoma multiforme. Acta Radiol 47:852–861
Chiba Y, Kinoshita M, Okita Y et al (2012) Use of (11)C-methionine PET parametric response map for monitoring WT1 immunotherapy response in recurrent malignant glioma. J Neurosurg 116:835–842
Popperl G, Kreth FW, Herms J et al (2006) Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med 47:393–403
Srinivasan R, Phillips JJ, Vandenberg SR et al (2010) Ex vivo MR spectroscopic measure differentiates tumor from treatment effects in GBM. Neuro-Oncology 12:1152–1161
Smith EA, Carlos RC, Junck LR et al (2009) Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. AJR 192:W45–W52
Boxerman JL, Ellingson BM, Jeyapalan S, et al (2014) Longitudinal DSC-MRI for distinguishing tumor recurrence from pseudoprogression in patients with a high-grade glioma. Am J Clin Oncol. doi:10.1097/COC.0000000000000156
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reardon, D.A., Okada, H. Re-defining response and treatment effects for neuro-oncology immunotherapy clinical trials. J Neurooncol 123, 339–346 (2015). https://doi.org/10.1007/s11060-015-1748-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1748-7