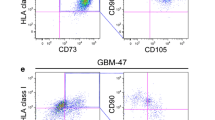
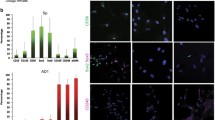
Abstract
Glioblastoma (GB) is a highly infiltrative tumor recurring in 90% of cases within a few centimeters of the resection cavity, even in cases of complete tumor resection and adjuvant chemo/radiotherapy. This observation highlights the importance of understanding this special zone of brain tissue surrounding the tumor. It is becoming clear that the nonneoplastic stromal compartment of most solid cancers plays an active role in tumor proliferation, invasion, and metastasis. Very little information, other than that concerning angiogenesis and immune cells, has been collected for stromal cells from GB. As part of a translational research program, we have isolated a new stromal cell population surrounding GB by computer-guided stereotaxic biopsies and primary culture. We named these cells GB-associated stromal cells (GASCs). GASCs are diploid, do not display the genomic alterations typical of GB cells, and have phenotypic and functional properties in common with the cancer-associated fibroblasts (CAFs) described in the stroma of carcinomas. In particular, GASCs express markers associated with CAFs such as fibroblast surface protein, alpha-smooth muscle actin (α-SMA), and platelet-derived growth factor receptor-beta (PDGFRβ). Furthermore, GASCs have a molecular expression profile different from that of control stromal cells derived from non-GB peripheral brain tissues. GASCs were also found to have tumor-promoting effects on glioma cells in vitro and in vivo. The isolation of GASCs in a tumor of neuroepithelial origin was unexpected, and further studies are required to determine their potential as a target for antiglioma treatment.





Similar content being viewed by others
Abbreviations
- CAFs:
-
Cancer-associated fibroblasts
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FCA:
-
Absolute fold change
- GASCs:
-
GB-associated stromal cells
- GB:
-
Glioblastoma
- HABS:
-
Human AB serum
- hMSCs:
-
Human mesenchymal stem cells
- IZ:
-
Interface zone
- NZ:
-
Necrotic zone
- PBZ:
-
Peripheral brain zone
- PDGFRβ:
-
Platelet-derived growth factor receptor-beta
- Q-PCR:
-
Real-time quantitative polymerase chain reaction
- α-SMA:
-
Alpha-smooth muscle actin
- TZ:
-
Tumor zone
References
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC–NCIC trial. Lancet Oncol 10:459–466
Giese A, Kucinski T, Knopp U et al (2004) Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol 66:351–360
Lefranc F, Brotchi J, Kiss R (2005) Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol 23:2411–2422
Liang BC, Thornton AF Jr, Sandler HM et al (1991) Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg 75:559–563
Tlsty TD, Coussens LM (2006) Tumor stroma and regulation of cancer development. Annu Rev Pathol 1:119–150
Ostman A, Augsten M (2009) Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr Opin Genet Dev 19:67–73
Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432:332–337
Shimoda M, Mellody KT, Orimo A (2010) Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol 21:19–25
Franco OE, Shaw AK, Strand DW et al (2010) Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 21:33–39
Xie Z (2009) Brain tumor stem cells. Neurochem Res 34:2055–2066
Chen R, Nishimura MC, Bumbaca SM et al (2010) A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell 17:362–375
Jain RK, di Tomaso E, Duda DG et al (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Yang I, Han SJ, Kaur G et al (2010) The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci 17:6–10
Sonabend AM, Rolle CE, Lesniak MS (2008) The role of regulatory T cells in malignant glioma. Anticancer Res 28:1143–1150
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Vindelov LL, Christensen IJ, Nissen NI (1983) A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 3:323–327
Roger M, Clavreul A, Venier-Julienne MC et al (2010) Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials 31:8393–8401
Hupe P, Stransky N, Thiery JP et al (2004) Analysis of array CGH data: from signal ratio to gain and loss of DNA regions. Bioinformatics 20:3413–3422
Clavreul A, Jean I, Preisser L et al (2009) Human glioma cell culture: two FCS-free media could be recommended for clinical use in immunotherapy. In Vitro Cell Dev Biol Anim 45:500–511
Glas M, Rath BH, Simon M et al (2010) Residual tumor cells are unique cellular targets in glioblastoma. Ann Neurol 68:264–269
Westermark B, Ponten J, Hugosson R (1973) Determinants for the establishment of permanent tissue culture lines from human gliomas. Acta Pathol Microbiol Scand A 81:791–805
Rutka JT, Giblin JR, Dougherty DY et al (1987) Establishment and characterization of five cell lines derived from human malignant gliomas. Acta Neuropathol 75:92–103
Gibbons HM, Hughes SM, Van Roon-Mom W et al (2007) Cellular composition of human glial cultures from adult biopsy brain tissue. J Neurosci Methods 166:89–98
Orimo A, Weinberg RA (2007) Heterogeneity of stromal fibroblasts in tumors. Cancer Biol Ther 6:618–619
Raica M, Cimpean AM (2010) Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals 3:572–599
Amillet JM, Ferbus D, Real FX et al (2006) Characterization of human Rab20 overexpressed in exocrine pancreatic carcinoma. Hum Pathol 37:256–263
Lee HS, Han J, Bai HJ et al (2009) Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J 276:4622–4635
Idbaih A, Carvalho Silva R, Criniere E et al (2008) Genomic changes in progression of low-grade gliomas. J Neurooncol 90:133–140
van Agthoven T, Sieuwerts AM, Meijer-van Gelder ME et al (2009) Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J Clin Oncol 27:542–549
Blaschuk OW, Devemy E (2009) Cadherins as novel targets for anti-cancer therapy. Eur J Pharmacol 625:195–198
Krishna K, Redies C (2009) Expression of cadherin superfamily genes in brain vascular development. J Cereb Blood Flow Metab 29:224–229
Teodorczyk M, Martin-Villalba A (2009) Sensing invasion: cell surface receptors driving spreading of glioblastoma. J Cell Physiol 222:1–10
Hayward SW, Wang Y, Cao M et al (2001) Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 61:8135–8142
Orimo A, Gupta PB, Sgroi DC et al (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348
Gonda TA, Varro A, Wang TC et al (2010) Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Semin Cell Dev Biol 21:2–10
Bauchet L, Mathieu-Daude H, Fabbro-Peray P et al (2010) Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316:1324–1331
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273
Phillips HS, Kharbanda S, Chen R et al (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173
Tso CL, Shintaku P, Chen J et al (2006) Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res 4:607–619
Ricci-Vitiani L, Pallini R, Larocca LM et al (2008) Mesenchymal differentiation of glioblastoma stem cells. Cell Death Differ 15:1491–1498
Gunther HS, Schmidt NO, Phillips HS et al (2008) Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene 27:2897–2909
Rieske P, Golanska E, Zakrzewska M et al (2009) Arrested neural and advanced mesenchymal differentiation of glioblastoma cells-comparative study with neural progenitors. BMC Cancer 9:54
Carro MS, Lim WK, Alvarez MJ et al (2010) The transcriptional network for mesenchymal transformation of brain tumours. Nature 463:318–325
Nakamizo A, Marini F, Amano T et al (2005) Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 65:3307–3318
Nakamura K, Ito Y, Kawano Y et al (2004) Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther 11:1155–1164
Kang SG, Shinojima N, Hossain A et al (2010) Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery 67:711–720
Mishra PJ, Humeniuk R, Medina DJ et al (2008) Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68:4331–4339
Acknowledgments
We thank neurosurgeons (Eric Lioret, CHU, Tours; Phong Dam Hieu, CHU, Brest; Abderrahmane Hamlat, CHU, Rennes) for supplying tumor samples, and the central committee of neuropathologists (Delphine Loussouarn, CHU, Nantes; Stephan Saikali, CHU, Rennes; Sophie Michalak, CHU, Angers; Isabelle Quintin, CHU, Brest). We also thank Emilie Munnier (Service d’Oncologie Médicale, CHRU, Tours), Jérôme Cayon (INSERM U646, Angers), Anne Bordron (Laboratoire de Thérapie Cellulaire, CHU, Brest), Lucie Karayan-Tapon (INSERM U935, Poitiers), Maryvonne Ardourel (Laboratoire de Neurobiologie, Orléans) and Lisa Oliver (INSERM U601, Nantes) for their help in the preparation of cell samples. We thank Pierre Legras and Jérôme Roux (Service Commun d’Animalerie Hospitalo-Universitaire, Angers), Catherine Guillet (Service Commun de Cytométrie et d’Analyse Nucléotidique, Angers), Marina Denyset, and the members of the Cancéropole Grand Ouest for allowing us to use their facilities. This work was supported by the French National Cancer Institute (INCa) and the Ligue Départementale de Lutte Contre le Cancer.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Clavreul, A., Etcheverry, A., Chassevent, A. et al. Isolation of a new cell population in the glioblastoma microenvironment. J Neurooncol 106, 493–504 (2012). https://doi.org/10.1007/s11060-011-0701-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0701-7