Abstract
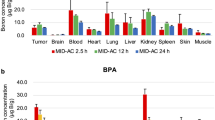
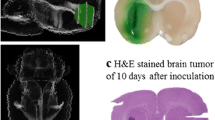
Boron neutron capture therapy (BNCT) is based on the nuclear capture and fission reactions that occur when non-radioactive 10B is irradiated with low energy thermal neutrons to produce α-particles (10B[n,α]7Li). Carboranylporphyrins are a class of substituted porphyrins containing multiple carborane clusters. Three of these compounds, designated H2TBP, H2TCP, and H2DCP, have been evaluated in the present study. The goals were two-fold. First, to determine their biodistribution following intracerebral (i.c.) administration by short term (30 min) convection enhanced delivery (CED) or sustained delivery over 24 h by Alzet™ osmotic pumps to F98 glioma bearing rats. Second, to determine the efficacy of H2TCP and H2TBP as boron delivery agents for BNCT in F98 glioma bearing rats. Tumor boron concentrations immediately after i.c. pump delivery were high and they remained so at 24 h. The corresponding normal brain concentrations were low and the blood and liver concentrations were undetectable. Based on these data, therapy studies were initiated at the Massachusetts Institute of Technology (MIT) Research Reactor (MITR) with H2TCP and H2TBP 24 h after CED or pump delivery. Mean survival times (MST) ± standard deviations of animals that had received H2TCP or H2TBP, followed by BNCT, were of 35 ± 4 and 44 ± 10 days, compared to 23 ± 3 and 27 ± 3 days, respectively, for untreated and irradiated controls. However, since the tumor boron concentrations of the carboranylporphyrins were 3–5× higher than intravenous (i.v.) boronophenylalanine (BPA), we had expected that the MSTs would have been greater. Histopathologic examination of brains of BNCT treated rats revealed that there were large numbers of porphyrin-laden macrophages, as well as extracellular accumulations of porphyrins, indicating that the seemingly high tumor boron concentrations did not represent the true tumor cellular uptake. Nevertheless, our data are the first to show that carboranyl porphyrins can be used as delivery agents for BNCT of an experimental brain tumor. Based on these results, we now are in the process of synthesizing and evaluating carboranylporphyrins that could have enhanced cellular uptake and improved therapeutic efficacy.





Similar content being viewed by others
References
Vicente MGH (2006) Boron in medicinal chemistry. Anti-cancer Agents Med Chem 6:73
Barth RF, Coderre JA, Vicente MGH, Blue TE (2005) Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 11:3987–4002
Sköld K, H-Stenstam B, Diaz AZ et al (2010) Boron neutron capture therapy for glioblastoma multiforme: advantage of prolonged infusion of BPA-f. Acta Neurol Scand 122:58–62
Sköld K, Gorlia T, Pellettieri L et al (2010) Boron neutron capture therapy for newly diagnosed glioblatoma multiforme: an assessment of clinical potential. Brit Radio 83:596–603
Miyatake S-I, Kawabata S, Yokoyama K et al (2009) Survival benefit of boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol 91:199–206
Kawabata S, Miyatake S-I, Kuroiwa T et al (2009) Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res 50:51–60
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concimitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase UUU study: 5-year analysis of the ORTC-NCIC trial. Lancet Oncol 10:459–466
Altieri S, Bortolussi S, Barth RF, Roveda L, Zonta A (2009) Thirteenth international congress on neutron capture therapy. Appl Radiat Isot 67:S1–378
Fairchild RG, Kahl SB, Laster BH et al (1990) In vitro determination of uptake, retention, distribution, biological efficacy, and toxicity of boronated compounds for neutron capture therapy: a comparison of porphyrins with sulfhydryl boron hydrides. Cancer Res 50:4860–4865
Hill JS, Kahl SB, Kaye AH et al (1992) Selective tumor uptake of a boronated porphyrin in an animal model of cerebral glioma. Proc Natl Acad Sci USA 89:1785–1789
Ceberg CP, Brun A, Kahl SB et al (1995) A comparative study on the pharmacokinetics and biodistribution of boronated porphyrin (BOPP) and sulfhydryl boron hydride (BSH) in the RG2 rat glioma model. J Neurosurg 83:86–92
Koo MS, Ozawa T, Santos RA et al (2007) Synthesis and comparative toxicology of a series of polyhedral borane anion-substituted tetraphenyl porphyrins. J Med Chem 50:820–827
Miura M, Joel DD, Smilowitz HM et al (2001) Biodistribution of copper carboranyltetraphenylporphyrins in rodents bearing an isogeneic or human neoplasm. J Neurooncol 52:111–117
Miura M, Micca PL, Fisher CD et al (1998) Evaluation of carborane-containing porphyrins as tumour targeting agents for boron neutron capture therapy. Br J Radiol 71:773–781
Miura M, Morris GM, Micca PL et al (2001) Boron neutron capture therapy of a murine mammary carcinoma using a lipophilic carboranyltetraphenylporphyrin. Radiat Res 155:603–610
Tibbitts J, Fike JR, Lamborn KR et al (1999) Toxicology of a boronated porphyrin in dogs. Photochem Photobiol 69:587–594
Tibbitts J, Sambol NC, Fike JR et al (2000) Plasma pharmacokinetics and tissue biodistribution of boron following administration of a boronated porphyrin in dogs. J Pharm Sci 89:469–477
Tsurubuchi T, Yamamoto T, Nakai K et al (2009) Intracellular uptake of a new boronated porphyrin EC032. Appl Radiat Isot 67:94–96
Viaggi M, Dagrosa MA, Longhino J et al (2004) Boron neutron capture therapy for undifferentiated thyroid carcinoma: preliminary results with the combined use of BPA and BOPP. Appl Radiat Isot 61:905–909
Wu H, Micca PL, Makar MS, Miura M (2006) Total syntheses of three copper (II) tetracarboranylphenylporphyrins containing 40 or 80 boron atoms and their biological properties in EMT-6 tumor-bearing mice. Bioorg Med Chem 14:5083–5092
Renner MW, Miura M, Easson MW, Vicente MGH (2006) Recent progress in the syntheses and biological evaluation of boronated porphyrins for boron neutron-capture therapy. Anticancer Agents Med Chem 6:145–157
Vicente MGH, Sibrian-Vazquez M (2010) Synthesis of boronated porphyrins and their application in BNCT. In: Kadish KM, Smith KM, Guilard R (eds) The handbook of porphyrin science, vol 4, chapter 18. World Scientific Publishers, Singapore, pp 191–248
Fabris C, Vicente MGH, Hao E et al (2007) Tumour-localizing and -photosensitising properties of meso-tetra(4-nido-carboranylphenyl)porphyrin (H2TCP). J Photochem Photobiol B 89:131–138
Soncin M, Friso E, Jori G et al (2008) Tumor-localizing and radiosensitising properties of meso-tetra(4-nido-carboranylphenyl)porphyrin (H2TCP). J Porphyr Phthalcocya 12:866–873
Vicente MGH, Nurco DJ, Shetty SJ et al (2002) Synthesis, dark toxicity and induction of in vitro DNA photodamage by a tetra(4-nido-carboranylphenyl)porphyrin. J Photochem Photobiol B 68:123–132
Vicente MGH, Shetty S, Wickramasinghe A, Smith KM (2000) Syntheses of carbon-carbon linked carboranylated porphyrins for application in boron neutron capture therapy. Tetrahedron Lett 41:7626–7627
Vicente MGH, Wickramasinghe A, Nurco DJ et al (2003) Synthesis, toxicity and biodistribution of two 5, 15-di[3, 5-(nido-carboranylmethyl)phenyl]porphyrins in EMT-6 tumor bearing mice. Bioorg Med Chem 11:3101–3108
Gottumukkala V, Ongayi O, Baker DG et al (2006) Synthesis, cellular uptake and animal toxicity of a tetra(carboranylphenyl)-tetrabenzoporphyrin. Bioorg Med Chem 14:1871–1879
Ongayi O, Gottumukkala V, Fronczek FR, Vicente MG (2005) Synthesis and characterization of a carboranyl-tetrabenzoporphyrin. Bioorg Med Chem Lett 15:1665–1668
Ozawa T, Afzal J, Lamborn KR et al (2005) Toxicity, biodistribution, and convection-enhanced delivery of the boronated porphyrin BOPP in the 9L intracerebral rat glioma model. Int J Radiat Oncol Biol Phys 63:247–252
Ozawa T, Santos RA, Lamborn KR et al (2004) In vivo evaluation of the boronated porphyrin TABP-1 in U-87 MG intracerebral human glioblastoma xenografts. Mol Pharm 1:368–374
Yang W, Barth RF, Adams DM et al (2002) Convection-enhanced delivery of boronated epidermal growth factor for molecular targeting of EGF receptor-positive gliomas. Cancer Res 62:6552–6558
Morrison PF, Chen MY, Chadwick RS et al (1999) Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol 277:1218–1229
Mardor Y, Rahav O, Zauberman Y et al (2005) Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res 65:6858–6863
Ferguson S, Lesniak MS (2007) Convection enhanced drug delivery of novel therapeutic agents to malignant brain tumors. Curr Drug Deliv 4:169–180
Sampson JH, Akabani G, Archer GE et al (2008) Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol 10:320–329
Kawabata S, Barth RF, Yang W, et al (2006) Evaluation of carboranylporphyrins as boron delivery agents for neutron capture therapy. In: Proceedings for the 12th international congress on neutron capture therapy for cancer. Takamatsu, Japan, pp 123–126
Bobadova-Parvanova P, Oku Y, Wickramasinghe A, Hall RW, Vicente MGH (2004) Ab initio and 1H-NMR study of the Zn(II) complexes of a nido- and a close-carboranylporphyrin. J Porphyr Phthalcocya 8:996–1006
Newcomb EW, Zugzog D (2009) The murine GL261 glioma experimental mode to assess novel brain tumor treatments, Chap. 12. CNS Cancer. Human press, New York, pp 227–241
Barth RF, Adams DM, Soloway AH et al (1991) Determination of boron in tissues and cells using direct-current plasma atomic emission spectroscopy. Anal Chem 63:890–893
Ko L, Koestner A, Wechsler W (1980) Morphological characterization of nitrosourea-induced glioma cell lines and clones. Acta Neuropathol 51:23–31
Barth RF, Kaur B (2009) Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol 94:299–312
Barth RF, Yang W, Rotaru JH et al (2000) Boron neutron capture therapy of brain tumors: enhanced survival and cure following blood-brain barrier disruption and intracarotid injection of sodium borocaptate and boronophenylalanine. Int J Radiat Oncol Biol Phys 47:209–218
Rogus RD, Harling OK, Yanch JC (1994) Mixed field dosimetry of epithermal neutron beams for boron neutron capture therapy at the MITR-II research reactor. Med Phys 21:1611–1625
Barth RF, Wu G, Yang W et al (2004) Neutron capture therapy of epidermal growth factor (+) gliomas using boronated cetuximab (IMC-C225) as a delivery agent. Appl Radiat Isot 61:899–903
Madsen RW, Moeschberger ML (1986) Statistical concepts. Prentice-Hall, Englewood Cliffs, NJ
Klein JP, Moeschberger ML (2003) Survival analysis techniques for censored and truncated data, 2nd edn. Springer, New York
Jori G, Soncin M, Friso E et al (2009) A novel boronated-porphyrin as a radio-sensitizing agent for boron neutron capture therapy of tumours: in vitro and in vivo studies. Appl Radiat Isot 67:321–324
Coderre JA, Glass JD, Fairchild RG et al (1987) Selective targeting of boronophenylalanine to melanoma in BALB/c mice for neutron capture therapy. Cancer Res 47:6377–6383
Shibata Y, Matsumura A, Yoshida F et al (1998) Cell cycle dependency of porphyrin uptake in a glioma cell line. Cancer Lett 129:77–85
Chandra S, Tjarks W, Lorey DR, Barth RF (2008) Quantitative subcellular imaging of boron compounds in individual mitotic and interphase human glioblastoma cells with imaging secondary ion mass spectrometry (SIMS). J Microsc 229:92–103
Smith DR, Chandra S, Coderre JA, Barth RF (1997) Quantitative ion microscopy imaging of boron-10 in rat brain tumor models for BNCT. In: Larsson B, Crawford J, Weinrich R et al (eds) Advances in neutron capture therapy, vol II, chemistry and biology. Elsevier Science B.V., Amsterdam, pp 308–314
Smith DR, Chandra S, Coderre JA, Morrison GH (1996) Ion microscopy imaging of 10B from p-boronophenylalanine in a brain tumor model for boron neutron capture therapy. Cancer Res 56:4302–4306
Barth RF, Yang W, Al-Madhoun AS, Johnsamuel J et al (2004) Boron-containing nucleosides as potential delivery agents for neutron capture therapy of brain tumors. Cancer Res 64:6287–6295
Acknowledgments
This paper is dedicated to Professor Otto Harling in recognition of his outstanding contributions to the field of BNCT research, and more specifically to his vision and foresight that made the Massachusetts Institute of Technology Research Reactor one of the leading facilities in the world to carry out BNCT studies. Sadly, such studies are no longer being carried out at this facility. We thank Ms. Michelle Van Fossen for expert secretarial assistance in the preparation of this manuscript and Dr. Michael Pennell, Division of Biostatistics, OSU, College of Public Health, for his helpful comments relating to statistical evaluation of the data. The studies described in this report were supported by N.I.H. grants R01 CA098902 (M.G.H.V.) and R01 CA098945 (R.F.B.), and the United States Department of Energy through the program of Innovations in Nuclear Infrastructure and Education, Office of Nuclear Energy, Science and Technology (contract no. DE-FG07-02ID14420DE-FG07-02, K14420), and the Office of Environmental and Biological Research (contract no. DE-FG02-02ER63358) (K.J.R. and P.J.B.). One of US (RFB) gratefully acknowledges support of The Ohio State University Department of Pathology for partial funding of the final stages of this study.
Conflicts of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawabata, S., Yang, W., Barth, R.F. et al. Convection enhanced delivery of carboranylporphyrins for neutron capture therapy of brain tumors. J Neurooncol 103, 175–185 (2011). https://doi.org/10.1007/s11060-010-0376-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0376-5