Abstract
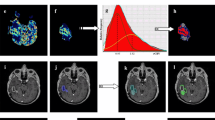
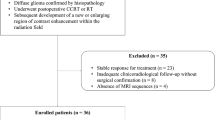
To analyse the role of MR diffusion-tensor imaging (DTI) and perfusion-weighted imaging (PWI) in characterising tumour boundaries in patients with glioblastoma multiforme. Seventeen patients with surgically treated WHO IV grade gliomas who were candidates for adjuvant chemo-radiotherapy were enrolled. Before (T0) and after radiation treatment (T1), they underwent DTI and PWI, and the apparent diffusion coefficient (ADC), fractional anisotropy (FA) and relative cerebral blood volume (rCBV) in the enhancing tumour, the hyperintense tissue adjacent to the enhancing tumour, and the normal-appearing white matter (NAWM) adjacent to the hyperintense areas were analysed. The enhancing tissue at T1 was retrospectively divided on the basis of whether or not it was also enhancing at T0. The controls were the corresponding contralateral areas, on which we normalized the rCBV values, calculating the rCBV ratio. In NAWM, we did not find any significant differences in FA, ADC or rCBV. In the hyperintense perilesional regions, FA was significantly lower and ADC significantly higher than in the unaffected contralateral tissue; there were no significant differences in the rCBV maps. The values of FA, ADC and rCBV in enhancing neoplastic tissue were all significantly different from those observed in the contralateral tissue. There was no significant difference in rCBV values between the areas enhancing at T0 and those not enhancing at T0 but enhancing at T1, which may indicate the neoplastic transformation of apparently normal brain tissue. DTI metrics identify ultrastructural changes in hyperintense perilesional areas, but these are not specific for neoplastic tissue. rCBV seemed to reflect an ultrastructural alteration that was not visible at T0, but became visible (as neoplastic progression) on conventional MR images at T1. These findings could help identify tissue at risk of tumour infiltration.








Similar content being viewed by others
References
Scarabino T, Popolizio T, Tosetti M, Montanaro D et al (2009) Phenylketonuria: white-matter changes assessed by 3.0-T magnetic resonance (MR) imaging, MR spectroscopy and MR diffusion. Radiol Med 114(3):461–474
Rizzo L, Crasto SG, Moruno PG et al (2009) Role of diffusion- and perfusion-weighted MR imaging for brain tumour characterisation. Radiol Med 114(4):645–659
Law M, Young RJ, Babb JS et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247(2):490–498
Kealey SM, Kim Y, Provenzale JM (2004) Redefinition of multiple sclerosis plaque size using diffusion tensor. Am J Roentgenol 183(2):497–503
Lu S, Ahn D, Johnson G, Law M et al (2004) Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 232(1):221–228
Sugahara T, Korogi Y, Kochi M et al (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. Am J Roentgenol 171(6):1479–1486
Macdonald DR, Cascino TL, Schold SC et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Manka C, Träber F, Gieseke J et al (2005) Three-dimensional dynamic susceptibility-weighted perfusion MR imaging at 3.0 T: feasibility and contrast agent dose. Radiology 234(3):869–877
Tanner SF, Cornette L, Ramenghi LA et al (2003) Cerebral perfusion in infants and neonates: preliminary results obtained using dynamic susceptibility contrast enhanced magnetic resonance imaging. Arch Dis Child Fetal Neonatal Ed 88(6):F525–F530
Wetzel SG, Cha S, Johnson G et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803
Streiner DL, Norman GR (1989) Health measurement scale. A practical guide to their development and use. Oxford University Press, New York
McKnight TR, von dem Bussche MH, Vigneron DB et al (2002) Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg 97:794–802
Kelly PJ, Daumas-Duport C, Kispert DB et al (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66(6):865–874
Shaw EG, Stieber V, Tatter S et al (2002) A Phase I dose escalating study of intensity modulated radiation therapy (IMRT) for the treatment of glioblastoma multiforme (GBM). Int J Radiat Oncol Biol Phys 54:206
Sinha S, Bastin ME, Whittle IR et al (2002) Diffusion tensor MR imaging of high-grade cerebral gliomas. Am J Neuroradiol 23(4):520–527
Stieltjes B, Schlüter M, Didinger B et al (2006) Diffusion tensor imaging in primary brain tumors: reproducible quantitative analysis of corpus callosum infiltration and contralateral involvement using a probabilistic mixture model. Neuroimage 31(2):531–542
Provenzale JM, McGraw P, Mhatre P et al (2004) Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 232(2):451–460
Kono K, Inoue Y, Nakayama K, Shakudo M et al (2001) The role of diffusion-weighted imaging in patients with brain tumors. Am J Neuroradiol 22(6):1081–1088
Lu S, Ahn D, Johnson G, Cha S et al (2003) Peritumoral diffusion tensor imaging in high-grade gliomas and metastatic brain tumors. Am J Neuroradiol 24:937–941
Zhou XJ, Yang S, Srinivasan G et al (2007) A study of glioma infiltration using fractional anisotropy and fiber coherence index. Proc Int Soc Mag Reson Med 15:344
Provenzale JM, Mukundan S, Barboriak DP (2006) Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 239(3):632–649
Kassner A, Annesley DJ, Zhu XP et al (2000) Abnormalities of the contrast re-circulation phase in cerebral tumors demonstrated using dynamic susceptibility contrast-enhanced imaging: a possible marker of vascular tortuosity. J Magn Reson Imaging 11:103–113
Barajas RF Jr, Hodgson JG, Chang JS et al (2010) Glioblastoma multiforme regional genetic and cellular expression patterns: influence on anatomic and physiologic MR imaging. Radiology 254(2):564–576
Murakami R, Sugahara T, Nakamura H et al (2007) Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 243(2):493–499
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stecco, A., Pisani, C., Quarta, R. et al. DTI and PWI analysis of peri-enhancing tumoral brain tissue in patients treated for glioblastoma. J Neurooncol 102, 261–271 (2011). https://doi.org/10.1007/s11060-010-0310-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0310-x