Abstract
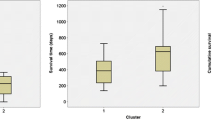
Perfusion estimates and microvascular leakage (MVL) were recently correlated with glioma angiogenesis and aggressiveness, but their role in predicting outcome of patients (pts) with unfavorable low-grade gliomas (ULGG) is unclear. Their prognostic value was then investigated, versus conventional factors such as age, neurological status, tumor size, and contrast enhancement (CE). Clinical and anatomical magnetic resonance imaging (MRI) criteria of a cohort of ULGG pts were prospectively evaluated. A dynamic T2*-weighted MR sequence was included to detect high-perfusion areas, using the maximal value of the relative cerebral blood volume (rCBV) estimate, and MVL. Conventional and microvascular characteristics were correlated with progression-free survival (PFS). Among the 46 pts included, the following features were present in 61%, 26%, 67%, and 26%, respectively: age ≥40 years, neurological deficits, tumor size ≥6 cm, and CE. High perfusion value was noted in 30% of cases and MVL in 52%. With median follow-up of 22 months (range 4–46 months), median PFS was 32 months [95% confidence interval (CI) 17–45 months]. On univariate analysis, CE, rCBV, and MVL were significantly correlated with PFS. On multivariate analysis, only CE and MVL were unfavorable factors, with hazard ratio of 3.0 and 7.3 and P value of 0.04 and 0.02, respectively. Different prognostic subgroups were identified, with 2-year PFS of 86%, 57%, and 19% for pts with no MVL, MVL without CE, and MVL with CE, respectively. MVL and CE seem to predict short-term outcome in ULGG pts.



Similar content being viewed by others
References
Lote K, Egeland T, Hager B et al (1997) Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol 15:3129–3140
Baumert BG, Stupp R (2008) Low-grade glioma: a challenge in therapeutic options: the role of radiotherapy. Ann Oncol 19(Suppl 7):vii217–vii222
Pignatti F, van den Bent M, Curran D et al (2002) Prognostic factors for survival in adults patients with cerebral low-grade glioma. J Clin Oncol 20:2076–2084
van den Bent MJ, Afra D, de Witte O et al (2005) Long term efficacy of early versus delayed radiotherapy for low-grade astrocytoma andoligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366:985–990
Kaloshi G, Benouaich-Amiel A, Diakite F et al (2007) Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology 68:1831–1836
Lang FF, Gilbert MR (2006) Diffusely infiltrative low-grade gliomas in adults. J Clin Oncol 24:1236–1245
Stupp R, Hegi ME, Gilbert MR et al (2007) Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol 25:4127–4136
Cao Y, Sundgren PC, Tsien CT et al (2006) Physiologic and metabolic magnetic resonance imaging in gliomas. J Clin Oncol 24:1228–1235
Knopp EA, Cha S, Johnson G et al (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Sadeghi N, Salmon I, Decaestecker C et al (2007) Stereotactic comparison among cerebral blood volume, methionine uptake, and histopathology in brain glioma. AJNR Am J Neuroradiol 28:455–461
Roberts HC, Roberts TPL, Brasch RC et al (2000) Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 21:891–899
Law M, Yang S, Babb J et al (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Gambarota G, Leenders W, Maass C et al (2008) Characterisation of tumour vasculature in mouse brain by USPIO contrast-enhanced MRI. Br J Cancer 98:1784–1789
Leach MO, Brindle KM, Evelhoch JL et al (2005) The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 92:1599–1610
Rosen BR, Belliveau JW, Vevea JM et al (1990) Perfusion imaging with NMR contrast agents. Magn Reson Med 14:249–265
Cao Y, Shen Z, Chenevert TL et al (2006) Estimate of vascular permeability and cerebral blood volume using Gd-DPTA contrast enhancement and dynamic T2*-weighted MRI. J Magn Reson Imaging 24:288–296
Boxerman JL, Schmainda KM, Weisskoff RM et al (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Kleihues P, Cavenee WK (eds) (2000) Pathology and genetics: tumours of the nervous system. In: WHO classification of tumors. IARC press, Lyon, France, pp 56–61
Law M, Brodsky J, Babb J et al (2007) High cerebral blood volume in human gliomas predicts deletion of chromosome 1p: preliminary results of molecular studies in gliomas with elevated perfusion. J Magn Reson Imaging 25:1113–1119
Quinn JA, Reardon DA, Friedman AH et al (2003) Phase II study of temozolomide in patients with progressive low-grade gliomas. J Clin Oncol 21:646–651
Law M, Oh S, Babb J et al (2006) Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Prediction of patient clinical response. Radiology 238:658–667
Law M, Young R, Babb J et al (2008) Gliomas: predicting time to progression or survival with CBV measurements at dynamic susceptibility-weighted contrast-enhance perfusion MR imaging. Radiology 247:490–498
Law M, Young R, Babb J et al (2006) Comparing perfusion metrics obtained from a single compartment versus pharmacokinetic modeling methods using dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 27:1975–1982
Lev MH, Vedolin L (2006) Permeability versus cerebral blood volume measurement in brain tumor evaluation: comparative clinical value and advice to authors. AJNR Am J Neuroradiol 27:418–419
Provenzale JM, Wang GR, Brenner T et al (2002) Comparison of permeability in high-grade and low-grade brain tumors using DSC MR imaging. AJR 178:711–716
Acknowledgments
We thank Lorna Saint Ange for editing and Schering-Plough S.A. for funding the major part of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dhermain, F., Saliou, G., Parker, F. et al. Microvascular leakage and contrast enhancement as prognostic factors for recurrence in unfavorable low-grade gliomas. J Neurooncol 97, 81–88 (2010). https://doi.org/10.1007/s11060-009-9992-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9992-3