Abstract
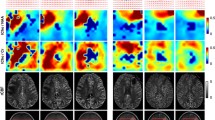
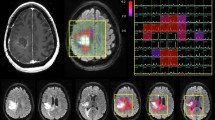
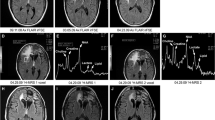
Objective Early prediction of imminent failure during chemotherapy for malignant glioma has the potential to guide proactive alterations in treatment before frank tumor progression. We prospectively followed patients with recurrent malignant glioma receiving tamoxifen chemotherapy using proton magnetic resonance spectroscopic imaging (1H-MRSI) to identify intratumoral metabolic changes preceding clinical and radiological failure. Methods We performed serial 1H-MRSI examinations to assess intratumoral metabolite intensities in 16 patients receiving high-dose oral tamoxifen monotherapy for recurrent malignant glioma (WHO grade III or IV) as part of a phase II clinical trial. Patients were followed until treatment failure, death, or trial termination. Results Patients were officially classified as responders (7 patients) or non-responders (9 patients) 8 weeks into treatment. At 8 weeks, responders and non-responders had different intratumoral intensities across all measured metabolites except choline. Beyond 8 weeks, metabolite intensities remained stable in all responders, but changed again with approaching disease progression. Choline, lipid, choline/NAA, and lactate/NAA were significantly elevated (P < 0.02), while creatine (P < 0.04) was significantly reduced, compared to stabilized levels on average 4 weeks prior to failure. Lactate was significantly elevated (P = 0.036) fully 8 weeks prior to failure. In one patient who was still responding to tamoxifen at the conclusion of the trial, metabolite intensities never deviated from 8-week levels for the duration of follow-up. Conclusions Characteristic global intratumoral metabolic changes, detectable on serial 1H-MRSI studies, occur in response to chemotherapy for malignant glioma and may predict imminent treatment failure before actual clinical and radiological disease progression.




Similar content being viewed by others
References
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomized trials. Lancet 359:1011–1018
Glioma Meta-Analysis Trialists (GMT) Group (2002) Chemotherapy for high grade glioma. Cochrane Database Syst Rev 4:CD003913
Hou LC, Veeravagu A, Hsu AR, Tse VCK (2006) Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus 20:E3
Simpson L, Galanis E (2006) Recurrent glioblastoma multiforme: advances in treatment and promising drug candidates. Expert Rev Anticancer Ther 6:1593–1607
Macdonald TR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
McKnight TR (2004) Proton magnetic resonance spectroscopic evaluation of brain tumor metabolism. Semin Oncol 31:605–617
Nelson SJ (2004) Magnetic resonance spectroscopic imaging: evaluating response to therapy for gliomas. IEEE Eng Med Biol Mag 23:30–39
Henson JW, Gonzalez RG (2004) Neuroimaging in glioma therapy. Expert Rev Neurother 4:665–671
Robertson JF (2004) Selective oestrogen receptor modulators/new anti-oestrogens: a clinical perspective. Cancer Treat Rev 30:695–704
O’Brian CA, Liskamp RM, Solomon DH, Weinstein IB (1985) Inhibition of protein kinase C by tamoxifen. Cancer Res 45:2462–2465
Horgan K, Cooke E, Hallett MB, Mansel RE (1986) Inhibition of protein kinase C mediated signal transduction by tamoxifen. Importance for anitumour activity. Biochem Pharmacol 35:4463–4465
Couldwell WT, Antel JP, Apuzzo MLJ, Yong VW (1990) Inhibition of growth of established human glioma lines by modulators of the protein kinase C second messenger system. J Neurosurg 73:594–600
Pollack IF, Randall MS, Kristofik MP, Kelly RH, Selker RG, Vertosick FT (1990) Effect of tamoxifen on DNA synthesis and proliferation of human malignant glioma lines in vitro. Cancer Res 50:7134–7138
Lien EA, Wester K, Lonning PE, Solheim E, Ueland PM (1991) Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer 63:641–645
Vertosick FT, Selker RG, Pollack IF, Arena V (1992) The treatment of intracranial malignant gliomas using orally administered tamoxifen therapy: preliminary results in a series of failed patients. Neurosurgery 30:897–903
Couldwell WT, Hinton DR, Surnock AA, DeGiorgio CM, Weiner LP, Apuzzo MLJ, Masri L, Law RE, Weiss MH (1996) Treatment of recurrent malignant gliomas with chronic oral high-dose tamoxifen. Clin Cancer Res 2:619–622
Chamberlain MC, Kormanik PA (1999) Salvage chemotherapy with tamoxifen for recurrent anaplastic astrocytomas. Arch Neurol 56:703–708
Brandes AA, Ermani M, Turazzi S, Scelzi E, Berti F, Amista P, Rotillo A, Licata C, Florentino MV (1999) Procarbazine and high-dose tamoxifen as a second-line regimen in high-grade gliomas: a phase II study. J Clin Oncol 17:645–650
Tang PA, Roldan G, Brasher PMA, Fulton D, Roa W, Murtha A, Cairncross JG, Forsyth PA (2006) A phase II study of carboplatin and chronic high-dose tamoxifen in patients with recurrent malignant glioma. J Neurooncol 78:311–316
Preul MC, Caramanos Z, Villemure J-G, Shenouda G, Leblanc R, Langleben A, Arnold DL (2000) Using proton magnetic resonance spectroscopic imaging to predict in vivo the response of recurrent malignant gliomas to tamoxifen chemotherapy. Neurosurgery 46:306–312
Nelson SJ, Huhn S, Vigneron DB, Day MR, Wald LL, Prados M, Chang S, Gutin PH, Sneed PK, Verhey L, Hawkins RA, Dillon WP (1997) Volume MRI and MRSI techniques for the quantitation of treatment response in brain tumors: presentation of a detailed case study. JMRI 7:1146–1152
Wald LL, Nelson SJ, Day MR, Noworolski SE, Henry RG, Huhn SL, Chang S, Prados MD, Sneed PK, Larson DA, Wara WM, McDermott M, Dillon WP, Gutin PH, Vigneron DB (1997) Serial proton magnetic resonance spectroscopy imaging of glioblastoma multiforme after brachytherapy. J Neurosurg 87:525–534
Graves EE, Nelson SJ, Vigneron DB, Verhey L, McDermott M, Larson D, Chang S, Prados MD, Dillon WP (2001) Serial proton MR spectroscopic imaging of recurrent malignant gliomas after gamma knife radiosurgery. AJNR Am J Neuroradiol 22:613–624
Zeng QS, Li CF, Zhang K, Liu H, Kang XS, Zhen JH (2007) Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neurooncol 84:63–69
Alexander A, Murtha A, Abdulkarim B, Mehta V, Wheatley M, Murray B, Riauka T, Hanson J, Fulton D, Urtasun R, McEwan A, Roa W (2006) Prognostic significance of serial magnetic resonance spectroscopies over the course of radiation therapy for patients with malignant glioma. Clin Invest Med 29:301–311
Lazareff JA, Gupta RK, Alger J (1999) Variation of post-treatment H-MRSI choline signal intensity in pediatric gliomas. J Neurooncol 41:291–298
Tzika AA, Zurakowski D, Poussaint TY, Goumnerova L, Astrakas LG, Barnes PD, Anthony DC, Billett AL, Tarbell NJ, Scott RM, Black PM (2001) Proton magnetic resonance spectroscopic imaging of child’s brain: the response of tumors to treatment. Neuroradiology 43:169–177
Murphy PS, Viviers L, Abson C, Rowland IJ, Brada M, Leach MO, Dzik-Jurasz ASK (2004) Monitoring temozolomide treatment of low-grade glioma with proton magnetic resonance spectroscopy. Br J Cancer 90:781–786
Balmaceda C, Critchell D, Mao X, Cheung K, Pannullo S, DeLaPaz RL, Shungu DC (2006) Multisection 1H magnetic resonance spectroscopic imaging assessment of glioma response to chemotherapy. J Neurooncol 76:185–191
Chinot O (2001) Chemotherapy for the treatment of oligodendroglial tumors. Semin Oncol 28:13–18
Nelson SJ, Vigneron DB, Dillon WP (1999) Serial evaluation of patients with brain tumors using volume MRI and 3D 1H-MRSI. NMR Biomed 12:123–138
Pirzkall A, McKnight TR, Graves EE, Carol MP, Sneed PK, Wara WW, Nelson SJ, Verhey LJ, Larson DA (2001) MR-spectroscopy guided target delineation for high grade gliomas. Int J Radiat Oncol Biol Phys 50:915–928
Nelson SJ, Graves E, Pirzkall A, Li X, Antiniw Chan A, Vigneron DB, McKnight TR (2001) In vivo molecular imaging for planning radiation therapy of gliomas: An application of 1H-MRSI. J Magn Reson Imaging 16:464–476
Graves EE, Nelson SJ, Vigneron DB, Chin C, Verhey L, McDermott M, Larson D, Sneed PK, Chang S, Prados MD, Lamborn K, Dylan WP (2000) A preliminary study of the prognostic value of proton magnetic resonance spectroscopic imaging in gamma knife radiosurgery of recurrent malignant gliomas. Neurosurgery 46:319–326
McKnight TR, von dem Bussche MH, Vigneron DB, Lu Y, Berger MS, McDermott MW, Dillon WP, Grave EE, Pirzkall A, Nelson SJ (2002) Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg 97:794–802
Waldrop SM, Davis PC, Padgett CA, Shapiro MB, Morris R (1998) Treatment of brain tumors in children is associated with abnormal MR spectroscopic ratios in brain tissue remote from the tumor site. AJNR Am J Neuroradiol 19:963–970
Miller BL, Chang L, Booth R, Ernst T, Cornford M, Nikas D, McBride D, Jenden DJ (1996) In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 58:1929–1935
Shimizu H, Kumabe T, Shirane R, Yoshimoto T (2001) Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. AJNR Am J Neuroradiol 21:659–665
Utriainen M, Komu M, Vuorinen V, Lehikoinen P, Sonninen P, Kurki T, Utriainen T, Rovainen A, Kalimo H, Minn H (2003) Evaluation of brain tumor metabolism with [11C] choline PET and 1H-MRS. J Neurooncol 62:329–338
Negendank WG, Sauter R, Brown TR, Evelhoch JL, Falini A, Gotsis ED, Heerschap A, Kamada K, Lee BC, Mengeot MM, Moser E, Padavic-Schaller KA, Sanders JA, Spraggins TA, Stillman AE, Terwey B, Vogl TJ, Wicklow K, Zimmerman RA (1996) Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg 84:449–458
Tate AR, Majos C, Moreno A, Howe FA, Griffiths JR, Arus C (2003) Automated classification of short echo time in vivo 1H brain tumor spectra: a multicenter study. Magn Reson Med 49:29–36
Howe FA, Barton SJ, Cudlip SA, Stubbs M, Saunders DE, Murphy M, Wilkins P, Opstad KS, Doyle VL, McLean MA, Bell BA, Griffiths JR (2003) Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 49:223–232
Birken DL, Oldendorf WH (1989) N-acteyl-l-aspartic acid: A literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev 13:23–31
Mangiardi JR (1995) Biochemistry and metabolism of brain tumors. In: Kaye AH, Laws ER (eds) Brain tumors: an encyclopedic approach. Churchill Livingstone, New York, pp 99–112
Kugel H, Heindel W, Ernestus RI, Bunke J, du Mesnil R, Friedmann G (1992) Human brain tumors: spectral patterns detected with localized H-1 MR spectroscopy. Radiology 183:701–709
Murphy PS, Rowland IJ, Viviers L, Brada M, Leach MO, Dzik-Jurasz AS (2003) Could assessment of glioma methylene lipid resonance by in vivo (1)H-MRS be of clinical value? Br J Radiol 76:459–463
Barba I, Cabanas ME, Arus C (1999) The relationship between nuclear magnetic resonance visible lipids, lipid droplets, and cell proliferation in culture C6 cells. Cancer Res 59:1861–1868
Baltuch GH, Dooley NP, Villemure JG, Yong VW (1995) Protein kinase C and growth regulation of malignant gliomas. Can J Neurol Sci 22:264–271
Baltuch GH, Couldwell WT, Villemure JG, Yong VW (1993) Protein kinase C inhibitors suppress cell growth in established and low-passage glioma cell lines. A comparison between staurosporine and tamoxifen. Neurosurgery 33:495–501
Lange T, Dydak U, Roberts TP, Rowley HA, Bjeljac M, Boesiger P (2006) Pitfalls in lactate measurements at 3T. AJNR Am J Neuroradiol 27:895–901
Kim JH, Chang KH, Na DG, Song IC, Kwon BJ, Han MH, Kim K (2006) 3T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequences. AJNR Am J Neuroradiol 27:1412–1418
Pfisterer WK, Hendricks WP, Scheck AC, Nieman RA, Birkner TH, Krampla WW, Preul MC (2007) Fluorescent in situ hybridization and ex vivo 1H magnetic resonance spectroscopic examinations of meningioma tumor tissue: is it possible to identify a clinically aggressive subset of benign meningiomas? Neurosurgery 61:1048–1059
Acknowledgements
This study was supported by funds from the Montreal Neurological Institute, including the Jeanne Timmins Costello Foundation, the Killam Foundation, and funds from the Barrow Neurological Institute, including the Barrow Neurological Foundation, and the Newsome Chair of Neurosurgery Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sankar, T., Caramanos, Z., Assina, R. et al. Prospective serial proton MR spectroscopic assessment of response to tamoxifen for recurrent malignant glioma. J Neurooncol 90, 63–76 (2008). https://doi.org/10.1007/s11060-008-9632-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9632-3