Abstract
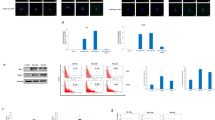
Given our previous findings that human cytomegalovirus (HCMV) nucleic acids and proteins are expressed in human malignant glioma in vivo, we investigated cellular signaling events associated with HCMV infection of human glioma and astroglial cells. HCMV infection caused rapid activation of the phosphatidylinositol-3 kinase (PI-3K) effector AKT kinase in human astro-glial and fibroblast cells, and induced tyrosine phosphorylation of phospholipase Cγ (PLCγ). Co-immunoprecipitation experiments revealed association of the p85 regulatory subunit of PI-3K with a high-molecular weight protein phosphorylated on tyrosine, following short-term exposure to HCMV. In contrast to a previous report, we were unable to confirm the identity of this high-molecular weight protein as being the epidermal growth factor receptor (EGFR). Stimulation of glioma and fibroblast cell lines over-expressing EGFR with HCMV (whole virus) or soluble glycoprotein B did not induce tyrosine phosphorylation of the receptor, as did the genuine ligand, EGF. Furthermore, we found that expression levels of the human ErbB1-4 receptors were not rate-limiting for HCMV infection. Dispensability of EGFR function during early HCMV infection was substantiated by demonstration of viral immediate early gene expression in cells lacking the EGFR gene, indicating that HCMV may promote oncogenic signaling pathways independently of EGFR activation. Among non-receptor cellular kinases, HCMV infection induced phosphorylation of focal adhesion kinase (FAK) Tyr397, which is indispensable for integrin-mediated cell migration and invasion. HCMV-induced FAK activation was paralleled by increased extracellular matrix-dependent migration of human malignant glioma but not normal astro-glial cells, suggesting that HCMV can selectively augment glioma cell invasiveness.




Similar content being viewed by others
References
Harkins L, Volk AL, Samanta M et al (2002) Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360:1557–1563
Cobbs CS, Harkins L, Samanta M et al (2002) Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62:3347–3350
Mitchell D, Xie W, Schmittling R et al (2007) Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology (In press)
Yu Y, Alwine JC (2002) Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J Virol 76:3731–3738
Zhu H, Shen Y, Shenk T (1995) Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J Virol 69:7960–7970
Streblow DN, Soderberg-Naucler C, Vieira et al (1999) The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511–520
Cinatl J, Scholz M, Kotchetkov R et al (2004) Molecular mechanisms of the modulatory effects of HCMV infection in tumor cell biology. Trends Mol Med 10:19–23
Boldogh I, AbuBakar S, Albrecht T (1990) Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science 247:561–564
Aaronson SA (1991) Growth factors and cancer. Science 254:1146–1153
Cooray S (2004) The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J Gen Virol 85:1065–1076
O’Shea CC (2005) DNA tumor viruses-the spies who lyse us. Curr Opin Genet Dev 15:18–26
Dawson CW, Tramountanis G, Eliopoulos et al (2003) Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem 278:3694–3704
Feire AL, Koss H, Compton T (2004) Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA 101:15470–15475
Wang X, Huang DY, Huong SM et al (2005) Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med 11:515–521
Wang X, Huong SM, Chiu et al (2003) Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456–461
Streblow DN, Vomaske J, Smith P et al (2003) Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J Biol Chem 278:50456–50465
Threadgill DW, Dlugosz AA, Hansen LA et al (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269:230–234
Di Fiore PP, Pierce JH, Fleming TP et al (1987) Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 51:1063–1070
Pruss RM, Herschman HR (1977) Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci USA 74:3918–3921
Sonoda Y, Ozawa T, Hirose Y et al (2001) Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res 61:4956–4960
Ruppert JM, Vogelstein B, Kinzler KW (1991) The zinc finger protein GLI transforms primary cells in cooperation with adenovirus E1A. Mol Cell Biol 11:1724–1728
Kraus MH, Popescu NC, Amsbaugh SC et al (1987) Overexpression of the EGF receptor-related proto-oncogene erbB-2 in human mammary tumor cell lines by different molecular mechanisms. Embo J 6:605–610
Jainchill JL, Aaronson SA, Todaro GJ (1969) Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol 4:549–553
Alimandi M, Romano A, Curia MC et al (1995) Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 10:1813–1821
Baulida J, Kraus MH, Alimandi et al (1996) All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem 271:5251–5257
Fedi P, Pierce JH, di Fiore PP et al (1994) Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol 14:492–500
Kraus MH, Fedi P, Starks V et al (1993) Demonstration of ligand-dependent signaling by the erbB-3 tyrosine kinase and its constitutive activation in human breast tumor cells. Proc Natl Acad Sci USA 90:2900–2904
Morgenstern JP, Land H (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res 18:3587–3596
Kraus MH, Yuasa Y, Aaronson SA (1984) A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci USA 81:5384–5388
Wang Z, La Rosa C, Maas R et al (2004) Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J Virol 78:3965–3976
Almeida-Porada G, Porada CD, Shanley JD et al (1997) Altered production of GM-CSF and IL-8 in cytomegalovirus-infected, IL-1-primed umbilical cord endothelial cells. Exp Hematol 25:1278–1285
Arbustini E, Grasso M, Diegoli M et al (1992) Histopathologic and molecular profile of human cytomegalovirus infections in patients with heart transplants. Am J Clin Pathol 98:205–213
Yarden Y, Schlessinger J (1987) Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry 26:1434–1442
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225
Schlaepfer DD, Hunter T (1998) Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol 8:151–157
Sabatier J, Uro-Coste E, Pommepuy I, Labrousse F et al (2005) Detection of human cytomegalovirus genome and gene products in central nervous system tumours. Br J Cancer 92:747–750
Valius M, Kazlauskas A (1993) Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell 73:321–334
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411:355–365
Isaacson MK, Feire AL, Compton T (2007) The epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J Virol. doi: 10.1128/JVI.00169-07
Demuth T, Berens ME (2004) Molecular mechanisms of glioma cell migration and invasion. J Neurooncol 70:217–228
Ritchie CK, Giordano A, Khalili K (2000) Integrin involvement in glioblastoma multiforme: possible regulation by NF-kappaB. J Cell Physiol 184:214–221
Sansal I, Sellers WR (2004) The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 22:2954–2963
Maher EA, Furnari FB, Bachoo RM (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15:1311–1333
Hsia DA, Mitra SK, Hauck et al (2003) Differential regulation of cell motility and invasion by FAK. J Cell Biol 160:753–767
Tsutsui Y, Kawasaki H, Kosugi I (2002) Reactivation of latent cytomegalovirus infection in mouse brain cells detected after transfer to brain slice cultures. J Virol 76:7247–7254
Singh SK, Hawkins C, Clarke et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Acknowledgments
We thank Robert Whitehead for EGFR-/- fibroblasts, Harvey Herschman for NR6 cells, Don J. Diamond and Zhongde Wang for purified gB protein, G. Yancey Gillespie for U251 glioma and J. Michael Ruppert for providing RK3E epithelial cell line. This study was supported by the UAB SPORE program in brain cancer (P50CA097247). Additional support by the Avon Breast Cancer Research Foundation is acknowledged (M.H.K.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cobbs, C.S., Soroceanu, L., Denham, S. et al. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness. J Neurooncol 85, 271–280 (2007). https://doi.org/10.1007/s11060-007-9423-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9423-2