Abstract
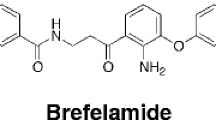
Nordy is a chiral compound synthesized based on the structure of a natural lipoxygenase (LO) inhibitor nordihydroguaiaretic acid (NDGA) from plants. The aim of the present study is to investigate the effect of Nordy on malignant human glioma cell responses to chemoattractants and growth promoting signals. We found that Nordy, in a non-cytotoxic concentration range, potently inhibited the chemotaxis and calcium flux of a human glioblastoma cell line U87 induced by a formylpeptide receptor (FPR) agonist, formyl-methionyl-leucyl-phenylalanine (fMLF) and epidermal growth factor (EGF). U87 cells treated by Nordy also showed a significantly impaired proliferation and expression of mRNA for vascular endothelial growth factor (VEGF) induced by fMLF. The chemotactic and proliferation responses of Nordy treated U87 cells to EGF were concomitantly diminished. Further experiments revealed that Nordy did not significantly affect FPR gene expression in U87 cells, but attenuated the activation of a plethora of signaling molecules including ERK1/2, p38, JNK, and Akt when the cells were stimulated by fMLF. EGF-induced EGF receptor phosphorylation was also inhibited in Nordy-treated U87 cells. Moreover, Nordy significantly reduced the tumorigenicity of U87 cells in nude mice. Our results suggest that Nordy is capable of inhibiting glioma cell responses to signals that promote cell motility, growth and production of VEGF. Thus, Nordy may constitute a molecular basis for the development of novel anti-cancer drugs.






Similar content being viewed by others
References
Schwartzbaum J, Ahlbom A, Malmer B et al (2005) Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res 65:6459–6465
Kleihues P, Cavenee WK (eds) (2000) Pathology and genetics of tumors of the nervous system. IARC Press, Lyon, p314
Bian XW, Chen JH, Jiang XF et al (2004) Angiogenesis as an immunopharmacologic target in inflammation and cancer. Int Immunopharmacol 4:1537–1547
Scott JN, Rewcastle NB, Brasher PM et al (1998) Long-term glioblastoma multiforme (GBM) survivors: a population-based study. Can J Neurol Sci 25:197–201
Arjona D, Bello MJ, Alonso ME et al. (2005) Molecular analysis of the EGFR gene in astrocytic gliomas: mRNA expression, quantitative-PCR analysis of non-homogeneous gene amplification and DNA sequence alterations. Neuropathol Appl Neurobiol 31:384–394
Le Y, Hu J, Gong W, Shen W et al (2000) Expression of functional formyl peptide receptors by human astrocytoma cell lines. Neuroimmunol 111:102–108
Zhou Y, Bian X, Le Y et al (2005) Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. J Natl Cancer Inst 97:823–835
Chen JH, Bian XW, Yao XH et al (2006) Nordy, a synthetic lipoxygenase inhibitor, inhibits the expression of formylpeptide receptor (FPR) and induces differentiation of malignant glioma cells. Biochem Biophys Res Commun 342:1368–1374
Bian XW, Jiang XF, Chen JH et al (2006) Increased angiogenic capabilities of endothelial cells from microvessels of malignant human gliomas. Int Immunopharmacol 6:90–99
Bian XW, Yang SX, Chen JH, et al (2007) Preferential expression of CXCR4 by more highly malignant human gliomas and association with poor patient survival. Neurosurg in press
Wang JM, Deng X, Gong W et al (1998) Chemokines and their role in tumor growth and metastasis. J Immunol Methods 220:1–17
Yang SX, Chen JH, Jiang XF et al (2005) Activation of chemokine receptor CXCR4 in malignant glioma cells promotes the production of vascular endothelial growth factor. Biochem Biophys Res Commun 335:523–528
Le Y, Wetzel MA, Shen W et al (2001) Desensitization of chemokine receptor CCR5 in dendritic cells at the early stage of differentiation by activation of formyl peptide receptors. Clin Immunol 99:365–372
Bian X, Du L, Shi J et al (2000) Correlation of bFGF, FGFR and VEGF expression with vascularity and malignancy of human astrocytomas. Analyt Quant Cytol Histol 22:267–274
Salcedo R, Wasserman K, Young HA et al (1999) Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1α. Am J Pathol 154:1125–1135
Farhadi MR, Capelle HH, Erber R et al (2005) Combined inhibition of vascular endothelial growth factor and platelet-derived growth factor signaling: effects on the angiogenesis, microcirculation, and growth of orthotopic malignant gliomas. J Neurosurg 102:363–370
Ikemoto S, Sugimura K, Kuratukuri K et al (2004) Antitumor effects of lipoxygenase inhibitors on murine bladder cancer cell line (MBT-2). Anticancer Res 24:733–736
Seufferlein T, Seckl MJ, Schwarz E et al (2002) Mechanisms of nordihydroguaiaretic acid-induced growth inhibition and apoptosis in human cancer cells. Br J Cancer 86:1188–1196
Lambert JD, Meyers RO, Timmermann BN et al (2001) Tetra-O-methylnordihydroguaiaretic acid inhibits melanoma in vivo. Cancer Lett 28:47–56
Tong WG, Ding XZ, Witt RC et al (2002) Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol Cancer Ther 1:929–935
Wilson DE, DiGianfilippo A, Ondrey FG et al (1989) Effect of nordihydroguaiaretic acid on cultured rat and human glioma cell proliferation. J Neurosurg 71:551–557
Ito H, Ueda H, Iwamoto I et al (2005) Nordihydroguaiaretic acid (NDGA) blocks the differentiation of C2C12 myoblast cells. J Cell Physiol 202:874–879
Bian X, Shi J, Xin R (1997) Effects of nordihydroguaiaretic acid on the growth and differentiation of SHG-44 glioma cell line. Zhonghua Bing Li Xue Za Zhi 26:285–288
Sun R, Iribarren P, Zhang N et al (2004) Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J Immunol 173:428–436
Yang D, Chertov O, Oppenheim JJ (2001) The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci 58:978–989
Arevalo-Rodriguez M, Pan X, Boeke JD et al (2004) FKBP12 controls aspartate pathway flux in Saccharomyces cerevisiae to prevent toxic intermediate accumulation. Eukaryot Cell 3:1287–1296
Migeotte I, Riboldi E, Franssen JD et al (2005) Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med 201:83–93
Czapiga M, Gao JL, Kirk A et al (2005) Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp Hematol 33:73–84
Rabiet MJ, Huet E, Boulay F (2005) Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol, 35:2486–2495
Gavins FN, Kamal AM, D’Amico M et al (2005) Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J 19:100–102
Becker EL, Forouhar FA, Grunnet ML et al (1998) Broad immunocytochemical localization of the formylpeptide receptor in human organs, tissues, and cells. Cell Tissue Res 292:129–135
Rescher U, Danielczyk A, Markoff A et al (2002) Functional activation of the formyl peptide receptor by a new endogenous ligand in human lung A549 cells. J Immunol 169:1500–1504
Wang ZG, Ye RD (2002) Characterization of two new members of the formyl peptide receptor gene family from 129S6 mice. Gene 299:57–63
Budd GC, Barnard EA, Porter C et al (1980) Fluorophosphate-sensitive esterases in mammalian liver: the radioautographic localization and measurement of fluorophosphate-reactive sites in adult rat liver. J Histochem Cytochem 28:533–542
Bjorndahl M, Cao R, Eriksson A et al (2004) Blockage of VEGF-induced angiogenesis by preventing VEGF secretion. Circ Res 94:1443–1450
Tokuda H, Kozawa O, Miwa M et al (2001) p38 mitogen-activated protein (MAP) kinase but not p44/p42 MAP kinase is involved in prostaglandin E1-induced vascular endothelial growth factor synthesis in osteoblasts. J Endocrinol 170:629–638
Haas-Kogan DA, Prados MD, Tihan T et al (2005) Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst 97:880–887
Nathoo N, Goldlust S, Vogelbaum MA (2004) Epidermal growth factor receptor antagonists: novel therapy for the treatment of high-grade gliomas. Neurosurg 54:1480–1488; discussion 1488–1489
Acknowledgements
The authors thank Dr J. J. Oppenheim for reviewing the manuscript; N. Dunlop for technical support; and Fogle C. and Nolan C. for secretarial assistance. This study was supported in part by fundings from UICC International Cancer Research Technology Transfer Award (No. 661/2002), National Natural Science Foundation of China (NSFC, No.30370552), Key Project of Chongqing Science and Technology Committee (CSTC-2005AA5007), and Federal funds from the National Cancer Institute, National Institutes of Health, under contract No. NO1-CO-12400. Chen JH is supported in part by a fellowship from the Office of the International Affairs, NCI, NIH, USA.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. All animals used in this research project were cared for and used humanely according to the following policies: The US Public Health Service Policy on Humane Care and Use of Animals; the Guide for the Care and Use of Laboratory Animals; and the US Government Principles for Utilization and Care of Vertebrate Animals Used in Testing, Research, and Train. The publisher or recipient acknowledges right of the US Government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
Rights and permissions
About this article
Cite this article
Chen, Jh., Yao, Xh., Gong, W. et al. A novel lipoxygenase inhibitor Nordy attenuates malignant human glioma cell responses to chemotactic and growth stimulating factors. J Neurooncol 84, 223–231 (2007). https://doi.org/10.1007/s11060-007-9369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9369-4