Abstract
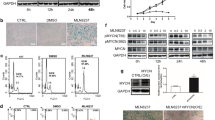
Cell division is an elemental process, and mainly consists of chromosome segregation and subsequent cytokinesis. Some errors in this process have the possibility of leading to carcinogenesis. Aurora-B is known as a chromosomal passenger protein that regulates cell division. In our previous studies of giant cell glioblastoma, we reported that multinucleated giant cells resulted from aberrations in cytokinesis with intact nuclear division occurring in the early mitotic phase, probably due to Aurora-B dysfunction. In this study, as we determined p53 gene mutation occurring in multinucleated giant cell glioblastoma, we investigated the role of Aurora-B in formation of multinucleated cells in human neoplasm cells with various p53 statuses as well as cytotoxity of glioma cells to temozolomide (TMZ), a common oral alkylating agent used in the treatment of gliomas. The inhibition of Aurora-B function by small-interfering (si)RNA led to an increase in the number of multinucleated cells and the ratios of G2/M phase in p53-mutant and p53-null cells, but not in p53-wild cells or the cells transduced adenovirally with wild-p53. The combination of TMZ and Aurora-B-siRNA remarkably inhibited the cell viability of TMZ-resistant glioma cells. Accordingly, our results suggested that Aurora-B dysfunction increases in the appearance of multinucleated cells in p53 gene deficient cells, and TMZ treatment in combination with the inhibition of Aurora-B function may become a potential therapy against p53 gene deficient and chemotherapeutic-resistant human gliomas.




Similar content being viewed by others
References
Albertson DG, Collins C, McCormick F, Gray JW (2003) Chromosome aberrations in solid tumors. Nat Genet 34:369–376
Jallepalli PV, Lenguer C (2001) Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer 1:109–117
Doxsey S (1998) The centrosome—a tiny organelle with big potential. Nat Genet 20:104–106
Bischoff JR, Plowman GD (1999) The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol 9:454–459
Giet R, Prigent C (1999) Aurora/Ipl 1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. Cell Sci 112:3591–3601
Nigg EA (2001) Cell division mitotic kinases as regulators of cell division and its checkpoints. Nature Rev Mol Cell Biol 2:21–32
Sugimoto K, Urano T, Zushi H, Inoue K, Tasaka H, Tachibana M, Dotsu M (2002) Molecular dynamics of Aurora-A kinase in living mitotic cells simultaneously visualized with histone H3 and nuclear membrane protein importinα. Cell Struct Funct 27:457–467
Berdnik D, Knoblich JA (2002) Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr Biol 12:640–647
Kufer TA, Nigg EA, Silljé HH (2003) Regulation of Aurora-A kinase on the mitotic spindle. Chromosoma 112:159–163
Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 17:3052–3065
Murata-Hori M, Wang Y-L (2002) Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J Cell Biol 159:45–53
Adams RR, Carmena M, Earnshaw WC (2001) Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol 11:49–54
Kimura M, Matsuda Y, Yoshioka T, Sumi N, Okano Y (1998) Identification of STK12/Aik2: a human gene related to aurora of Drosophila and yeast IPL1. Cytogenet Cell Genet 82:147–152
Zhao X, He M, Wan D, Ye Y, He Y, Han L, Guo M, Huang Y, Qin W, Wang MW, Chong W, Chen J, Zhang L, Yang N, Xu B, Wu M, Zuo L, Gu J (2003) The minimum LOH region defined on chromosome 17p13.3 in human hepatocellular carcinoma with gene content analysis. Cancer Lett 190:221–232
Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y (1998) Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res 58:4811–4816
Kimura M, Matsuda Y, Yoshioka T, Okano Y (1999) Cell Cycle-dependent expression and centrosome localization of a third human Aurora/Ipl1-related protein kinase, AIK3. J Biol Chem 274: 7368–7378
Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, Ohgaki H (2006) Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol 65: 846–854
Meyer-Puttlitz B, Hayashi Y, Waha A (1997) Molecular genetic analysis of giant cell glioblastomas. Am J Pathol 151:853–857
Muller W, Slowik F, Firsching R (1987) Contribution to the problem of giant cell astrocytomas. Neurosurg Rev 10:213–219
Palma L, Celli P, Maleci A (1989) Malignant monstrocellular brain tumours. A study of 42 surgically treated cases. Acta Neurochir 97:17–25
Peraud A, Watanabe K, Plate KH, Yoneyama Y, Kleihues P, Ohgaki H (1997) p53 mutations versus EGF receptor expression in giant cell glioblastomas. J Neuropathol Exp Neurol 56(11):1236–1241
Giangaspero F, Doglioni C, Rivano MT (1987) Growth fraction in human brain tumors defined by the monoclonal antibody Ki-67. Acta Neuropathol 74:179–182
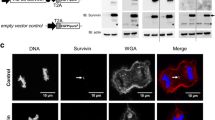
Fujita M, Mizuno M, Nagasaka T, Wakabayashi T, Maeda K, Ishii D, Arima T, Kawajiri A, Inagaki M, Yoshida J (2004) Aurora-B dysfunction of multinucleated giant cells in glioma detected by site-specific phosphorylated antibodies. J Neurosurg 101:1012–1017
Maeda K, Mizuno M, Wakabayashi T, Takasu S, Nagasaka T, Inagaki M, Yoshida J (2003) Morphological assessment of the development of multinucleated giant cells by using mitosis-specific phosphorylated antibodies. J Neurosurg 98:854–859
Tada M, Iggo RD, Waridel F, Nozaki M, Matsumoto R, Sawamura Y, Shinohe Y, Ikeda J, Abe H (1997) Reappraisal of p53 mutations in human malignant astrocytic neoplasms by p53 functional assay: comparison with conventional structural analyses. Mol Carcinog 18:171–176
Levine AJ (1997) p53, the cellar gatekeeper for growth and division. Cell 88:323–331
Helton ES, Chen X (2006) p53 modulation of the DNA damage response. J Cell Biochem 1 Oct 9 (in press)
Asaoka K, Tada M, Sawamura Y, Ikeda J, Abe H (2000) Dependence of efficient adenoviral gene delivery in malignant glioma cells on the expression levels of the Coxsackievirus and adenovirus receptor. J Neurosurg 92:1002–1008
Mondal AM, Chinnadurai S, Datta K, Chauhan SS, Sinha S, Chattopadhyay P (2006) Identification and functional characterization of a novel unspliced transcript variant of HIC-1 in human cancer cells exposed to adverse growth conditions. Cancer Res 66: 10466–10477
Okamura H, Yoshida K, Morimoto H, Hanaji T (2005) PTEN expression elicited by EGR-1 transcription factor in calyculin A-induced apoptotic cells. J Cell Biochem 94(1):117–125
Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM (2003) The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 161:281–294
Yamamoto S, Yoshida Y, Aoyagi M, Ohno K, Hirakawa K, Hamada H (2002) Reduced transduction efficiency of adenoviral vectors expressing human p53 gene by repeated transduction into glioma cells in vitro. Clin Cancer Res 8:913–921
Fults D, Brockmeyer D, Tullous MW, Pedone CA, Cawthon RM (1992) p53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Res 52:674–679
Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, Yoshida J (2005) IFN-β down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res 65:7573–7579
Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M (1998) AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J 17:667–676
Cox LS, Lane DP (1995) Tumor suppressors, kinases, clamps how p53 regulates the cell cycle in response to DNA damage. BioEssays 17:501–508
Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ Jr (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and CADD45 is detective in ataxia-telangiectasia. Cell 71:587–597
Lanni JS, Jacks T (1998) Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol 18:1055–1064
Minn AJ, Boise LH, Thompson CB (1996) Expression Bcl-x and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev 10:2621–2631
Harvey M, Sands AT, Weiss RS, Hegi ME, Wiseman RW, Pantazis P, Giovanella BC, Tainsky MA, Bradley A, Donehower LA (1993) In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 8:2457–2467
Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B (1999) 14–3-3σ is required to prevent mitotic catastrophe after DNA damage. Nature (London) 401:616–620
Vogel C, Kienitz A, Hofmann I, Müller R, Bastians H (2004) Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene 23:6845–6853
Huang MC, Kubo O, Tajika Y (1996) A clinico-immunohistochemical study of giant cell glioblastoma. Noshuyo Byori 13:11–16
Katayama H, Brinkley WR, Sen S (2003) The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev 22(4):451–464
Acknowledgements
We thank Professor Hirofumi Hamada, Department of Molecular Medicine, Sapporo Medical University, for providing adenoviral vectors. This manuscript was supported in part by Grants-in-Aid for Scientific Research 16209044 and 16390408 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuno, T., Natsume, A., Katsumata, S. et al. Inhibition of Aurora-B function increases formation of multinucleated cells in p53 gene deficient cells and enhances anti-tumor effect of temozolomide in human glioma cells. J Neurooncol 83, 249–258 (2007). https://doi.org/10.1007/s11060-007-9335-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9335-1