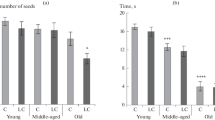
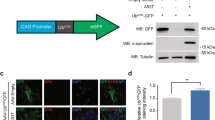
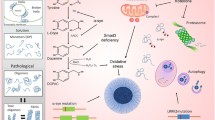
The pathomorphological signs of Parkinson’s disease (PD), an untreatable neurodegenerative condition, include the formation of pathological α-synuclein-positive inclusions in neurons. Studies using a model of the preclinical stage of PD in middle-aged and elderly rats based on increasing suppression of brain proteasome activity, along with analysis by light and confocal microscopy, addressed the presence and locations of pathological α-synuclein-positive inclusions in dopaminergic neurons in the compact part of the substantia nigra (SNc) and olfactory bulb. In the model of BP, α-synuclein aggregates were located in the cytoplasm of dopaminergic neurons in the olfactory bulb and the cytoplasm and nuclei of dopaminergic neurons in the SNc. A characteristic feature of α-synuclein pathology in aging rats was deposition of larger α-synuclein aggregates in the bodies of nerve cells in these locations as compared with middle-aged rats.
Similar content being viewed by others
References
I. N. Abdurasulova, I. V. Ekimova, A. V. Matsulevich, et al., “Impairments to nonassociative learning in rats in conditions of an experimental model of the preclinical stage of Parkinson’s disease,” Dokl. Akad. Nauk., 476, No. 3, 353–356 (2017).
I. V. Ekimova, V. V. Simonova, M. A. Guzeev, et al., “Changes in the characteristics of sleep in a model of the preclinical stage of Parkinson’s disease in rats created by weakening the activity of the ubiquitin- proteasome system of the brain,” Zh. Evolyuts. Biokhim. Fiziol., 52, No. 6, 413–422 (2016).
H. Bernheimer, W. Birkmayer, O. Hornykiewicz, et al., “Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations,” J. Neurol. Sci, 20, No. 4, 415–455 (1973).
M. Bisaglia, S. Mammi, and L. Bubacco, “Structural insights on physiological functions and pathological effects of α-synuclein,” FASEB J., 23, No. 2, 329–340 (2009).
H. Braak, E. Ghebremedhin, U. Rаb, et al., “Stages in the development of Parkinson’s disease-related pathology,” Cell Tissue Res., 318, No. 1, 121–134 (2004).
M. B. Fares, N. Ait-Bouziad, I. Dikiy, et al., “The novel Parkinson’s disease linked mutation G51D attenuates in vitro aggregation and membrane binding of α-synuclein, and enhances its secretion and nuclear localization in cells,” Hum. Mol. Genet., 23, No. 17, 4491– 4509 (2014).
K. A. Fujita, M. Ostaszewski, Y. Matsuoka, et al., “Integrating pathways of Parkinson’s disease in a molecular interaction map,” Mol. Neurobiol., 49, No. 1, 88–102 (2014).
B. I. Giasson, J. E. Duda, I. V. Murray, et al., “Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions,” Science, 290, No. 5493, 985–989 (2000).
J. V. Hindle, “Ageing, neurodegeneration and Parkinson’s disease,” Age Ageing, 39, No. 2, 156–161 (2010).
K. S. P. McNaught, D. P. Perl, A. L. Brownell, and C. W. Olanow, “Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease,” Ann. Neurol., 56, No. 1, 149–162 (2004).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, SDG (1998).
D. V. Plaksina, M. V. Chernyshev, M. N. Karpenko, et al., “Experimental modeling of a preclinical Parkinson’s disease stage in rats by intranasal lactacystin administration,” Neurodeg. Dis., 17, Suppl. 1, 1655 (2017).
A. H. Schapira, “Recent developments in biomarkers in Parkinson disease,” Curr. Opin. Neurol., 26, No. 4, 395 (2013).
Y. Tanaka, S. Engelender, S. Igarashi, et al., “Inducible expression of mutant α-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis,” Hum. Mol. Genet., 10, No. 9, 919–926 (2001).
T. Uchihara and B. I. Giasson, “Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies,” Acta Neuropathol., 131, No. 1, 49–73 (2016).
E. A. Waxman and B. I. Giasson, “A novel, high-effi ciency cellular model of fi brillar α-synuclein inclusions and the examination of mutations that inhibit amyloid formation,” J. Neurochem., 113, No. 2, 374–388 (2010). 17. J. L. Webb, B. Ravikumar, J. Atkins, et al., “α-Synuclein is degraded by both autophagy and the proteasome,” J. Biol. Chem., 278, No. 27, 25009–25013 (2003).
S. Yu, X. Li, G. Liu, et al., “Extensive nuclear localization of α-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody,” J. Neurosci., 145, No. 2, 539–555 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 104, No. 6, pp. 709–716, June, 2018.
Rights and permissions
About this article
Cite this article
Plaksina, D.V., Ekimova, I.V. Age-Related Features of α-Synuclein Pathology in the Brain on Modeling the Preclinical Stage of Parkinson’s Disease in Rats. Neurosci Behav Physi 50, 109–114 (2020). https://doi.org/10.1007/s11055-019-00875-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00875-0