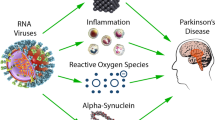
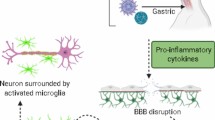
Parkinson’s disease (PD) is a multifactorial progressive neurodegenerative disease characterized by predominant degeneration of dopaminergic neurons in the substantia nigra. Neuroinflammation is one of the key components of the pathogenesis of PD, though the mechanisms initiating the inflammatory process and the triggers launching the irreversible neuroinflammatory process in patients with PD thus far remain unstudied. The present review addresses the role of infection-related factors in the etiology of PD. We evaluate the question of whether PD is the result of prior viral or bacterial infections due to the action of endotoxins on brain cells initiating the development of the inflammatory process in the CNS. Some cellular and animal models of PD of the infection type are presented and the molecular mechanisms of the development of neuroinflammation and neurodegeneration in these models are laid out. The final part of the review contains an analysis of reports, including those from the authors of this review, on the creation of valid models of the clinical and preclinical stages of PD in animals based on the proteasome inhibitor lactacystin, a metabolite of the soil bacterium Streptomyces sp.
Similar content being viewed by others
References
E. I. Gusev, A. B. Gekht, G. R. Popov, et al., Parkinson’s Disease. Clinical Aspects, Diagnosis, and Treatment of Neurodegenerative Diseases: Basic and Applied Aspects, M. V. Ugryumov (ed.), Nauka, Moscow (2010), pp. 52–86.
I. V. Ekimova, D. V. Plaksina, K. V. Lapshina, et al., “Pathological and compensatory processes in a new model of the preclinical stage of Parkinson’s disease in rats,” Acta Naturae, Spec. Iss., No. 1, 50 (2016).
I. V. Ekimova, V. V. Simonova, M. A. Guzeev, et al., “Changes in sleep characteristics in a model of the preclinical stage of Parkinson’s disease in rats based on weakening of the activity of the ubiquitin-proteasome system of the brain,” Zh. Evolyuts. Biokhim. Fiziol., 52, No. 6, 413–422 (2016).
S. N. Illarioshkin, “The course of Parkinson’s disease and approaches to the early diagnosis,” in: Parkinson’s Disease and Motor Disorders. Guidelines for Doctors: Proc. 2nd Nat. Congress on Parkinson’s Disease and Motor Disorders, S. N. Illarioshkin and O. S. Levin (eds.), Moscow (2011), pp. 41–47.
I. V. Milyukhina, M. N. Karpenko, A. A. Timofeeva, et al., “The role of inflammation and the pathogenesis of Parkinson’s disease,” Nevrol. Zh., 18, No. 3, 51–55 (2013).
Yu. F. Pastukhov, “Changes in the characteristics of paradoxical sleep – an early sign of Parkinson’s disease,” Zh. Vyssh. Nerv. Deyat., 63, No. 1, 75–85 (2013).
Yu. F. Pastukhov, I. V. Ekimova, and A. V. Chesnokova, “Molecular mechanisms of the pathogenesis of Parkinson’s disease and the potentials of preventive therapy,” in: Neurodegenerative Diseases – from Genome to the Whole Body. Part I. Motor Function and its Regulation in Health and Pathology, M. V. Ugryumov (ed.), Nauchnyi Mir, Moscow (2014), pp. 316–355.
Yu. F. Pastukhov, V. V. Simonova, M. A. Guzeev, and I. V. Ekimova, “Molecular mechanisms of sleep impairment at the initial stage of neurodegeneration induced by proteasomal dysfunction,” Acta Naturae, Spec. Iss., No. 1, 52 (2016).
Yu. F. Pastukhov, V. V. Simonova, M. V. Chernyshev, et al., “Signs of sleep impairment and behavior signaling the initial stage of neurodegenerative in a model of Parkinson’s disease,” Zh. Evolyuts. Biokhim. Fiziol., 53, No. 5, 380–384 (2017).
Yu. F. Pastukhov and A. Yu. Chesnokova, “α-Synuclein in the pathogenesis of Parkinson’s disease and other neurodegenerative diseases,” in: Neurodegenerative Diseases: Basic and Applied Aspects, M. V. Ugryumov (ed.), Nauka, Moscow (2010).
Yu. F. Pastukhov, A. Yu. Chesnokova, A. A. Yakimchuk, et al., “Changes in sleep in degeneration of the substantia nigra induced by the proteasome inhibitor lactacystin,” Ros. Fiziol. Zh., 96, No. 12, 1190–1202 (2010).
D. V. Plaksina, I. V. Ekimova, M. N. Karpenko, and Yu. F. Pastukhov, “Assessment of the functional state of the nigrostrial system of the brain in an experimental model of the preclinical stage of Parkinson’s disease in rats,” Zh. Evolyuts. Biokhim. Fiziol., 53, No. 5, 370–374 (2017).
M. V. Ugryumov, “Translated, personalized, and prophylactic medicine as the basis for the battle with neurodegenerative diseases,” in: Neurodegenerative Diseases – from Genome to the Whole Body, Nauchnyi Mir, Moscow (2014), pp. 316–355.
H. H. Balfour, S. K. Dunmire, and K. A. Hogquist, “Infectious mononucleosis,” Clin. Transl. Immunology, 4, No. 2, 33 (2015).
L. L. Barnes, A. W. Capuano, A. E. Aiello, et al., “Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals,” J. Infect. Dis., 211, No. 2, 230–237 (2015).
E. Bentea, L. Verbruggen, and A. Massie, “The proteasome inhibition model of Parkinson’s disease,” J. Parkinsons Dis., 7, No. 1, 31–63 (2017).
H. Braak, E. Ghebremedhin, U. Rub, et al., “Stages in the development of Parkinson’s disease related pathology,” Cell Tissue Res., 318, No. 1, 121–134 (2004).
X. L. Bu, X. Wang, Y. Xiang, et al., “The association between infectious burden and Parkinson’s disease: a case-control study,” Parkinsonism Relat. Disord., 21, No. 8, 877–881 (2015).
A. Cagnin, M. Kassiou, S. R. Meikle, and R. B. Banati, “Positron emission tomography imaging of neuroinflammation,” Neurotherapeutics, 4, No. 3, 443–452 (2007).
G. Çamci and S. Oğuz, “Association between Parkinson’s disease and Helicobacter pylori,” J. Clin. Neurology, 12, No. 2, 147–150 (2016).
P. M. Carvey, Q. Chang, J. W. Lipton, and Z. Ling, “Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson’s disease,” Front. Biosci., 8, 826–837 (2003).
A. Castano, A. J. Herrera, J. Cano, and A. Machado, “Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system,” J. Neurochem., 70, No. 4, 1584–1592 (1998).
G. Chapman, B. L. Beaman, D. A. Loeffler, et al., “In situ hybridization for detection of nocardial 16S rRNA: reactivity within intracellular inclusions in experimentally infected cynomolgus monkeys – and in Lewy body-containing human brain specimens,” Exp. Neurol., 184, No. 2, 715–725 (2003).
A. Charlett, R. J. Dobbs, S. M. Dobbs, et al., “Parkinsonism: siblings share Helicobacter pylori seropositivity and facets of syndrome,” Acta Neurol. Scand., 99, No. 1, 26–35 (1999).
A. Ciechanover and Y. T. Kwon, “Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies,” Exp. Mol. Med., 47, No. 3, е147 (2015).
T. Cross, “Aquatic actinomycetes: A critical survey of the occurrence, growth and role of actinomycetes in aquatic habitats,” J. Appl. Bacteriol., 50, No. 3, 397–423 (1981).
G. Deretzi, J. Kountouras, S. A. Polyzos, et al., “Gastrointestinal immune system and brain dialogue implicated in neuroinflammatory and neurodegenerative diseases,” Curr. Mol. Med., 11, No. 8, 696–707 (2011).
D. T. Dexter and P. Jenner, “Parkinson disease: From pathology to molecular disease mechanisms,” Free Radic. Biol. Med., 62, 132–144 (2013).
S. M. Dobbs, R. J. Dobbs, C. Weller, and A. Charlett, “Link between Helicobacter pylori infection and idiopathic parkinsonism,” Med. Hypotheses, 55, No. 2, 93–98 (2000).
C. T. M. Dow, “M. paratuberculosis and Parkinson’s disease – is this a trigger,” Med. Hypotheses, 83, No. 6, 709–712 (2014).
D. Ebrahimi-Fakhari, L. Wahlster, and P. J. McLean, “Protein degradation pathways in Parkinson’s disease: curse or blessing,” Acta Neuropathol., 124, No. 2, 153–172 (2012).
F. Fang, K. Wirdefeldt, A. Jacks, et al., “CNS infections, sepsis and risk of Parkinson’s disease,” Int. J. Epidemiol., 41, No. 4, 1042–1049 (2012).
G. Fenteany and S. L. Schreiber, “Lactacystin, proteasome function, and cell fate,” J. Biol. Chem., 273, No. 15, 8545–8548 (1998).
F. Fornai, P. Lenzi, M. Gesi, et al., “Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition,” J. Neurosci., 23, 8955–8966 (2003).
D. M. Forton, J. M. Allsop, I. J. Cox, et al., “A review of cognitive impairment and cerebral metabolite abnormalities in patients with hepatitis C infection,” AIDS, 19, 53–63 (2005).
H. M. Gao, J. Jiang, B. Wilson, et al., “Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease,” J. Neurochem., 81, No. 6, 1285–1297 (2002).
D. A. Gayle, Z. Ling, C. Tong, et al., “Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-α, interleukin-1β, and nitric oxide,” Dev. Brain Res., 133, No. 1, 27–35 (2002).
C. H. Hawkes, K. Del Tredici, and H. Braak, “Parkinson’s disease: a dual-hit hypothesis,” Neuropathol. Appl. Neurobiol., 33, No. 6, 599–614 (2007).
N. M. Joseph, B. N. Harish, S. Sistla, et al., “Streptomyces bacteremia in a patient with actinomycotic mycetoma,” J. Infect. Dev. Ctries., 4, No. 4, 249–252 (2010).
H. S. Jung, M. M. Ehlers, H. Lombaard, et al., “Etiology of bacterial vaginosis and polymicrobial biofilm formation,” Crit. Rev. Microbiol., 30, 1–17 (2017).
N. Kadoguchi, H. Kimoto, R. Yano, et al., “Failure of acute administration with proteasome inhibitor to provide a model of Parkinson’s disease in mice,” Metab. Brain. Dis., 23, 147–154 (2008).
S. Kohbata, and K. Shimokawa, “Circulating antibody to Nocardia in the serum of patients with Parkinson’s disease,” Adv. Neurology, 60, 355–357 (1992).
J. Konieczny, A. Czarnecka, T. Lenda, et al., “Chronic L-DOPA treatment attenuates behavioral and biochemical deficits induced by unilateral lactacystin administration into the rat substantia nigra,” Behav. Brain Res., 261, 79–88 (2014).
S. J. Kwon, T. B. Ahn, M. Y. Yoon, and B. S. Jeon, “BV-2 stimulation by lactacystin results in a strong inflammatory reaction and apoptotic neuronal death in SH-SY5Y cells,” Brain Res., 1205, 116–121 (2008).
E. Lahner, B. Annibale, and G. Delle Fave, “Systematic review: Helicobacter pylori infection and impaired drug absorption,” Aliment. Pharmacol. Ther., 29, No. 4, 379–386 (2009).
E. Lahner, C. Virili, M. G. Santaguida, et al., “Helicobacter pylori infection and drugs malabsorption,” World J. Gastroenterol., 20, No. 30, 10331–10337 (2014).
T. Laskus, M. Radkowski, D. M. Adair, et al., “Emerging evidence of hepatitis C virus neuroinvasion,” AIDS, 19, 140–144 (2005).
H. J. Lee, S. M. Baek, D. H. Ho, et al., “Dopamine promotes formation and secretion of non-fibrillar alpha-synuclein oligomers,” Exp. Mol. Med., 43, 4, 216–222 (2011).
Z. Ling, D. A. Gayle, S. Y. Ma, et al., “In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain,” Mov. Disord., 17, No. 1, 116–124 (2002).
D. A. Loeffler, D. M. Camp, S. Qu, et al., “Characterization of dopamine-depleting activity of Nocardia asteroides strain GUH-2 culture filtrate on PC12 cells,” Microb. Pathog., 37, No. 2, 73–85 (2004).
A. B. Manning-Bog, S. H. Reaney, V. P. Chou, et al., “Lack of nigrostriatal pathology in a rat model of proteasome inhibition,” Ann. Neurol., 60, No. 2, 256–260 (2006).
C. N. Martyn and C. Osmond, “Parkinson’s disease and the environment in early life,” J. Neurol. Sci, 132, No. 2, 201–206 (1995).
O. Marques and T. F. Outeiro, “Alpha-synuclein: from secretion to dysfunction and death,” Cell Death Dis., 3, e350 (2012).
B. N. Mathur, M. D. Neely, M. Dyllick-Brenzinger, et al., “Systemic administration of a proteasome inhibitor does not cause nigrostriatal dopamine degeneration,” Brain Res., 1168, 83–89 (2007).
R. M. McManus and M. T. Heneka, “Role of neuroinflammation in neurodegeneration: new insights,” Alzheimers Res. Ther., 9, No. 1, 14 (2017).
K. S. McNaught, L. M. Bjorklund, R. Belizaire, et al., “Proteasome inhibition causes nigral degeneration with inclusion bodies in rats,” Neuroreport, 13, No. 11, 1437–1441 (2002).
K. S. McNaught, R. Belizaire, O. Isacson, et al., “Altered proteasomal function in sporadic Parkinson’s disease,” Exp. Neurol., 179, No. 1, 38–46 (2003).
K. S. McNaught, D. P. Perl, A. L. Brownell, and C. W. Olanow, “Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease,” Ann. Neurol., 56, No. 1, 149–162 (2004).
J. J. Neher, U. Neniskyte, T. Hornik, and G. C. Brown, “Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo,” Glia, 62, No. 9, 1463–1475 (2014).
C. Niu, J. Me, Q. Pan, and X. Fu, “Nigral degeneration with inclusion body formation and behavioral changes in rats after proteasomal inhibition,” Stereotact. Funct. Neurosurg., 87, No. 2, 69–81 (2009).
C. Noelker, L. Morel, T. Lescot, et al., “Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease,” Sci. Rep., 3, 1393 (2013).
A. Ogata, K. Tashiro, S. Nukuzuma, et al., “A rat model of Parkinson’s disease induced by Japanese encephalitis virus,” J. Neurovirol., 3, No. 2, 141–147 (1997).
E. Okun, K. J. Griffioen, and M. P. Mattson, “Toll-like receptor signaling in neural plasticity and disease,” Trends Neurosci., 34, No. 5, 269–281 (2011).
Y. Ouchi, T. Kanno, H. Okada, et al., “Presynaptic and postsynaptic dopaminergic binding densities in the nigrostriatal and mesocortical systems in early Parkinson’s disease: A double-tracer positron emission tomography study,” Ann. Neurol., 46, No. 5, 723–731 (1999).
Y. Ouchi, E. Yoshikawa, Y. Sekine, et al., “Microglial activation and dopamine terminal loss in early Parkinson’s disease,” Ann. Neurol., 57, No. 2, 168–175 (2005).
D. V. Plaksina, M. V. Chernyshev, M. N. Karpenko, et al., “Experimental modeling of a preclinical Parkinson’s disease stage in rats by intranasal lactacystin administration,” Neurodegen. Dis. (Suppl), 17, No. 1, 1655 (2017).
A. Priyadarshi, S. A. Khuder, E. A. Schaub, and S. S. Priyadarshi, “Environmental risk factors and Parkinson’s disease: a metaanalysis,” Environ. Res., 86, No. 2, 122–127 (2001).
S. Sadasivan, B. Sharp, S. Schultz-Cherry, and R. J. Smeyne, “Synergistic effects of influenza and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: experimental evidence for the multi-hit hypothesis,” Parkinson’s Dis., 3, No. 1, 18 (2017).
M. H. Savolainen, K. Albert, M. Airavaara, and T. T. Myohanen, “Nigral injection of a proteasomal inhibitor, lactacystin, induces widespread glial cell activation and shows various phenotypes of Parkinson’s disease in young and adult mouse,” Exp. Brain Res., 1–14 (2017).
A. H. Schapira, M. W. Cleeter, J. R. Muddle, et al., “Proteasomal inhibition causes loss of nigral tyrosine hydroxylase neurons,” Ann. Neurol., 60, No. 2, 253–255 (2006).
S. A. Staras, S. C. Dollard, K. W. Radford, et al., “Seroprevalence of cytomegalovirus infection in the United States, 1988–1994,” Clin. Infect. Dis., 43, No. 9, 1143–1151 (2006).
A. H. Tan, S. Mahadeva, C. Marras, et al., “Helicobacter pylori infection is associated with worse severity of Parkinson’s disease,” Parkinsonism Relat. Disord., 21, No. 3, 221–225 (2015).
H. Tomoda and S. Omura, “Lactacystin, a proteasome inhibitor: discovery and its application in cell biology,” Yakugaku Zasshi, 120, No. 10, 935–949 (2000).
S. Toovey, S. S. Jick, and C. R. Meier, “Parkinson’s disease or Parkinson symptoms following seasonal influenza,” Influenza Other Respir. Viruses, 5, No. 5, 328–333 (2011).
H. H. Tsai, H. H. Liou, C. H. Muo, et al., “Hepatitis C virus infection as a risk factor for Parkinson disease A nationwide cohort study,” Neurology, 86, No. 9, 840–846 (2016).
J. Y. Wang, J. Y. Wang, J. Y. Wang, et al., “Ethanol modulates induction of nitric oxide synthase in glial cells by endotoxin,” Life Sci., 63, No. 17, 1571–1583 (1998).
J. M. Woulfe, M. T. Gray, D. A. Gray, et al., “Hypothesis: a role for EBV-induced molecular mimicry in Parkinson’s disease,” Parkinsonism Relat. Disord., 20, No. 7, 685–694 (2014).
W. Y. Wu, K. H. Kang, S. S. Chen, et al., “Hepatitis C virus infection: a risk factor for Parkinson’s disease,” J. Viral. Hepat., 22, No. 10, 784–791 (2015).
B. Y. Zeng, S. Bukhatwa, A. Hikima, et al., “Reproducible nigral cell loss after systemic proteasomal inhibitor administration to rats,” Ann. Neurol., 60, No. 2, 248–252 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 103, No. 8, pp. 841–853, August, 2017.
Rights and permissions
About this article
Cite this article
Karpenko, M.N., Muruzheva, Z.M., Pestereva, N.S. et al. An Infection Hypothesis of Parkinson’s Disease. Neurosci Behav Physi 49, 555–561 (2019). https://doi.org/10.1007/s11055-019-00769-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00769-1