Abstract
Catastrophic cases of methane explosion during exploratory drilling within the Bełchatów ortholignite deposit have led to testing for methane in other Polish ortholignite mining areas, as well as in the Złoczew deposit, where it is planned to begin mining operations. Initial tests have shown Złoczew lignite to have the highest methane content among the Polish deposits so far studied, comparable with lignite from the Bełchatów deposit, with a methane capacity in excess of 2.5 dcm3/kg at a pressure of 10 bar. Based on the computed values of the Langmuir constant, a determination was made of the quantity of methane that can be desorbed from a pressure of 10 bar to 1 bar, as well as the residual methane content. For all of the tested samples, the residual methane content is between 30 and 50% of the sorption capacity at a pressure of 10 bar. The thermal sorption equations were used to compute values of the limiting isosteric heat of adsorption. Higher values of the heat of adsorption at zero surface capacity may indicate the presence of a small quantity of micro-pores. In the case of the samples with the highest sorption capacity, the limiting isosteric heats of adsorption are low, indicating a low proportion of micro-pores in the lignite. This was confirmed by tests of nitrogen adsorption at 77 K. The proportion of micro-pores in the studied lignites is 2–3%, while the dominant pore fraction is the meso-pores, which in lignite from the Złoczew and Bełchatów deposits account for 50–66% of total pores. It is concluded that the significant adsorption of methane in the ortholignite occurs chiefly in meso-pores because of compression of the gas under increased orogenic pressure. A link is made between the higher methane-bearing capacity of the ortholignite deposits and the degree of gelification of the huminite components, based on simple statistical correlations between the methane sorption capacity and the content of humic gelified maceral. The results concerning methane sorption in lignite from the Złoczew deposit have enabled a preliminary classification of the methane-bearing capacity of Polish ortholignite deposits, which may also be of significance for similar deposits in other countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natural process of coalification of rocks of organic origin is accompanied by the emission of gases (Orem and Finkelman 2003). This is a process that depends on a number of factors, the most important of which include the type of initial material, the conditions under which it accumulated, the temperature, pressure, and geological time. Because of these geological factors, regardless of the degree of the coalification, coal becomes a natural collector of the produced gases. An estimation of the quantity of these gases is of great importance for the safety of mining operations and in the case of strata containing methane, for the recovery and utilization of that gas (Flores 2014).
The methane occurring in coal-bearing formations is produced by microbial processes—divided into early and late and thermogenic processes. At an early stage of the coalification of plant material (peat formation and diagenesis), methane is produced by microorganisms (Flores 2014). This is a natural process related chiefly to the presence of methane in peat, lignite and in subbituminous coal (Mayumi et al. 2016). Despite occurrences of microbial CH4 which are common in coal deposits (Strąpoć et al. 2011), rarely observed in lignite deposits is the late microbial process, where methane-producing microbial consortia of methanogens and bacteria enter coal strata with rainwater and form methane in the reduction conditions (Gründger et al. 2015). These processes are distinct from the thermogenic production of methane, which takes place in the later stages of coalification. This process has a markedly higher yield than those taking place in the earlier phases, because of which the content of methane in subbituminous and bituminous coals is significantly higher (Miller 2005).
According to the international coal classification, the Polish lignite deposits contain low-rank fossil fuels of the ortholignite class (UN-ECE/ENERGY/50, ISO 11760:2004). The principal form of methane occurring in strata of this type of coals, as in the case of medium- and high-rank coals (which have higher degrees of coalification), is adsorbed methane. It is bound physicochemically to the coal substance, and it would appear to be located in micro-pores within the coal (e.g., Czerw 2011; Czerw et al. 2016; Kudasik et al. 2017). The coal also contains free methane, which chiefly fills meso- and macro-pores and fissures in the coal and in the barren rocks. The methane dissolved in water within the deposits is of relatively low importance.
There is no comprehensive research on ortholignite porosity. One of the reasons is the unidentified lithotypes on an international scale so far, but few studies indicate that the porosity of different lithotypes of this coal reaches 0.0143 cm3/g (Henc et al. 2018). Detritic lignite seems to be the most porous.
The quantity of methane adsorbed in a coal stratum is determined by a number of factors, which may be divided into two broad groups: (1) factors determining the gas capacity (the sorption capacity) of the coal, which include the stratum’s degree of coalification, petrographic composition, moisture, content of mineral substance, the geothermal temperature of the rocks, and the deposit pressure; and (2) factors affecting the actual quantity of methane in the coal stratum, i.e., its methane-bearing capacity, which include the geological structure (the region’s lithology and tectonics). Because of these factors, the quantities of methane found in coal deposits are highly variable and often difficult to determine.
The methane-bearing capacity of ortholignite methane is much smaller than of more highly coalified formations. The reason of this is declining efficiency of microbiological processes and increasing categenetic efficiency. Still, they are big enough of to cause evidence of this is provided by the catastrophic methane explosions which occurred during prospective drilling at the Bełchatów open cast mine in Poland in 2001, the methane explosion disaster at the Kaławsk open-cast mine (in 1971). The widely reported incidents at lignite mines in other countries are as follows: in the ortholignite and metalignite deposits at Powder River in Wyoming and Montana, where the methane-bearing capacity is estimated at 1–3.5 m3/t and the total quantity of methane present is at around 2 × 1018 m3. Another lignite basin where significant quantities of methane have led to a catastrophic explosion is that of the Previdza region of Slovakia, where in 2009 a mixture of methane and coal dust exploded in the Handlova mine. An explosion of a similar type occurred in the Soma deep mine in Turkey in 2004. The above information was taken from the local daily press.
In Poland, as in many other coal-producing countries, the presence of methane in coal deposits is viewed primarily as a source of danger in mining operations. Methane content has already been studied in all of the currently mined ortholignite deposits: Konin, Adamów, Turów and Bełchatów-Szczerców (Macuda and Zawisza 2007; Macuda et al. 2011). Among the deposits where mining is planned to begin in the near future, preliminary studies of methane content have been carried out in the Złoczew region. This ortholignite deposit has of the highest methane contents of all of those studied in Poland.
The aim of the study was to determine the sorption uptake capacity of seven coal samples from the Szczerców deposit in relation to petrographic composition. The obtained results were also discussed with the results of studies of other Polish lignite deposits, which were published earlier by the co-authors.
Geological Structure of Złoczew Lignite Deposit
The Złoczew deposit is located approximately 50 km west of the currently worked open-cast mines belonging to KWB Bełchatów (Bełchatów and Szczerców), in the Kleszczów valley in central Poland (Fig. 1).
A detailed analysis of the lignite deposit was recently carried out in accordance with European Union principles (Hycnar et al. 2015), which took into account, among other factors, the petrological characteristics of coal. This is required by Instruction UN-ECE/ENERGY/50, which is applied here, and by the ISO 11760 international standard based on it.
The deposit is found in Łódzkie province, south of the town of Złoczew. In geological terms, it is situated in the southwestern part of the Łódź basin within the central Polish synclinorium. It is within the Złoczew tectonic valley, which at present forms the western part of the Kleszczów valley, where the Bełchatów and Szczerców ortholignite deposits are currently mined (Kasiński et al. 2010). The depth of the Złoczew valley (to Mesozoic sediments) is estimated at approximately 200–250 m and locally as much as 350 m. It is filled mainly with Neogene and Quaternary sediments (down to 350 m, average 180 m). Directly below the Cenozoic sediments are Jurassic and Cretaceous calcareous structures and their weathered forms.
The area of the Złoczew lignite deposit, within the boundaries of the currently planned mining area, forms a narrow strip between 1.5 and 4.0 km in width, running from southwest to northeast. According to GPS measurement carried out on the mound, its coordinates in the WGS84 system are B = 52° 28′ 30.89019″, L = 21° 02′ 02.97764″, H = 138.90 m.
The principal rocks of value contained in the deposit are a series of coal-bearing sediments from the Lower and Middle Miocene. The coal formation also contains locally, in the lower part, Paleocene formations such as weathered Mesozoic structures and partially preserved Oligocene sediments with a thin lignite stratum (Peryt and Piwocki 2004).
The profile of the coal-bearing sediments in this deposit comprises a series of complexes (Borowicz et al. 2007). The oldest is the sub-coal complex (Ucc; Fig. 2) formed from weathered Mesozoic calcareous rocks, sandy Oligocene sediments, as well as carbonaceous loams, silts and sands from the Early Miocene. Above this is a coal complex (Cc), which consists of several coal strata (3–5), interspersed with loamy and sandy strata and marl. This coal complex is divided into sub-complexes—loamy carbonaceous (CCc) and loamy sandy (CSc), which consist chiefly of loamy and sandy sediments. The youngest complex is a Pleistocene sedimentary series (Q) consisting of clay till together with rocks of aqueous and glacial origin. This complex consists mainly of plastic and impermeable rocks, isolating the free gas from emission to atmosphere.
Geological cross section of the Złoczew deposit, showing division into complexes (according Borowicz et al. 2007). Key: Q—Quaternary, CSc—loamy sandy complex, CCc—loamy carbonaceous complex, Cc—coal complex, Ucc—sub-coal complex
The maximum thickness of the coal complex (Cc) is estimated to be 130 m in the deepest part of the valley. It contains a 100-meter section consisting of several coal strata, of which the largest has a thickness of approximately 40 m (Borowicz et al. 2007; Sawicki 2010). The total geological quantity of lignite in the Złoczew deposit is estimated at 485,622,000 Mg (Sawicki 2010).
Experimental Measurement of Adsorbed Methane in the Złoczew Ortholignite Deposit
Methodology
Elemental analyses for H and C were obtained using the Carlo Erba EA 1108 elemental analyser. The moisture content was determined in accordance with the procedure set forth in the standard PN-80/G-04511. Ash content was established in accordance with PN-80/G-04512. The determination of the sulfur content was carried out using a method according to Polish Standard PN-81/G-04514.02. The oxygen content was computed as the remainder of 100%, taking into account the moisture and ash content.
Observations of the structural-texture features and macerals composition of the samples of coal and petrographic investigations of the polished sections (a transverse section) were performed with the polarized light microscope Axioplan (Zeiss-Opton, Oberkochen, Germany) for the reflected light, respectively. Measurements of the random reflectance (Ror ) of coal were made with the reflectometer Axioplan MPM-400 photometer in standard conditions (ISO 7404-5). In addition, ortholignite was observed in blue fluorescent reflected light using the Schött filters. The petrographic analysis was performed in accordance with ICCP regulations (international Committee for Coal and Organic Petrology). The quantitative petrographic analysis was performed by converting 500 intersection points.
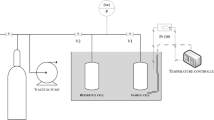
The sorption tests were performed using a volumetric apparatus (Fig. 3). Sorption measurement at elevated pressures by the manometric method involves the decompression of gas with specified pressure and volume from the reference cell (dosing space) to a sample cell containing a sorbent. The precision of measurement depends on the accuracy of the pressure measurements, the quality of the calibration, the sorbent density, and—most importantly—the stability of the temperature (Sakurovs et al. 2009; Gensterblum et al. 2010). Pressure measurements were taken with an S-10 pressure traducer (WIKA), operating in the range 0–100 bar, with BFSL (Best Fit Straight Line) accuracy class 0.25%. The design of the measurement device ensures thermal stability of both the ampoule and the dosing unit at identical temperature with accuracy of 0.1 K (the temperature was recorded using TC Pt 100 equipment).
Real (helium) coal density was determined using the automated apparatus Accu Pyc 1330 (Micrometrics).
The Petrographic Test Material
Tests were carried out on seven samples of ortholignite from drilling point Z-88, located in the eastern part of the deposit, where the thickness of the coal complex was approximately 110 meters. Samples weighing about 0.5 kg and representing the main lithotypes in the deposit were taken using a systematic method from depths of 220.5–305.5 m from the more industrially valuable coal strata in the deposit (Table 1).
The lignite in the Złoczew deposit is humic coal, as indicated by its lithological and petrographic composition (Tables 1 and 2). An analysis performed at more than 50 drilling points near the central axis of the deposit has shown that the majority of the lignite (approximately 60%) is stratified xylodetritic coal containing 10–30% xylites (X10-30D) and stratified detroxylitic coal (30%) with similar xylite content, but with loamy and calcareous mineral impurities (X25-40D). The remaining 10% of the average lithological composition consists of detritic coal, often with small quantities of xylites (up to 10%). The average total xylite content in lignite from this deposit is approximately 23% (unpublished research report).
The petrographic composition of the studied ortholignite reflects the average proportions of lithotypes in the structure of the deposit. The dominant macerals are those of the huminite group (61.0–82.2% by volume; Table 2). There are also large quantities of macerals of the liptinite group (4.9–17.3% by volume) and of the inertinite group (3.0–21.9%). The petrographically determined quantities of mineral material were variable and generally small, but with an extreme value in sample 30. The averaged values range from 0.6 to 29.6% by volume (Table 2). The high carbonate content comes from the limestones surroundings of the coal deposit and the layers of the lake chalk in the deposit (Wagner 2007).
Among the macerals of the huminite group, dominant are attrinite (3.3–33.7% by volume, but mostly above 15%) and densinite (0.6–37.7%, average 15.1%). There are also numerous component macerals of xylites, such as textoulminite (2.8–18.3%) and euulminite (8.0–22.6%). Quantities of textinite in the studied lignite are lower (0.0–8.2%). This petrographic composition, with a large quantity of gelified macerals as well as a significant content of gelinite (0.2–5.1% by volume), accurately reflects the lithology of the studied ortholignite and its degree of gelification, which is determined to be medium to high (Table 1).
Attrinite is found in the studied lignite in the form of a small-grained background rock with variable particle diameters, mostly up to several μm. In the less gelified strata (lithotypes)—samples 6, 33 and 42—it forms a homogenized mixture of weakly gelified humus microparticles, with small quantities of liptodetrinite, inertodetrinite and minerals, predominantly loamy minerals and calcite (sample 30). A characteristic feature of this maceral is the dense network of micro-cracks, identified with exogenous cleavage formed in the early stage of coalification (secondary, diagenetic gelification).
Similar in appearance is densinite. This is a strongly homogenized basic coal material, structurally uniform (samples 22, 33, 42 and 57), having the character of basic background rock, and resulting from the binding of small coal particles by highly dispersed humic gel. The appearance of this maceral reflects a process of diagenetic coalification (geochemical gelification).
The densinite in the studied lignite is strongly cracked, to a much greater degree than the attrinite. These cracks, being of the nature of exogenous cleavage, appear to be a result of geochemical coalification (Stach et al. 1982), as is clearly visible in the tectonic trough depression deposits of the ortholignite (e.g., Widera 2013, 2016).
The content of macerals of the liptinite group is due chiefly to the presence of liptodetrinite (2.9–7.0% by volume), as well as the presence in the petrographic composition of sporinite (0.6–1.2%) and similar quantities of other macerals of the same group (Table 2). The contents of resinite (0.4–9.8%) and cutinite (0.0–0.5%), found chiefly in attrinite and densinite, indicate the locally allochthonous nature of the lignite.
Among the macerals of the inertinite group, relatively common are funginite (1.4–6.4% by volume) and particularly inertodetrinite (3.0–21.9%), confirming the hydrated conditions of sedimentation of the organic matter in this deposit. Contents of fusinite and semifusinite are small, at less than 1% by volume (except in the case of samples 51 and 57, where their combined content was in the range 9.4–11.5%).
The Chemical Nature of the Lignite
The total moisture content (analyzed in accordance with an International Standard ISO 331: 1983) of the lignite is in range from 43.2 to 52.1% by weight. Air-dried lignite (40 °C) contains 7.7–10.2% moisture by weight (Table 3). The ash content (ISO 1171:1997) after combustion of the lignite is variable, but is generally low (8.3–13.7% by weight), except in sample 30, where it reaches 43.6%.
The results of the elemental analysis (ISO 609:1996, ISO 333:1996) of the lignite samples were as follows: Cdaf 66.4–68.7%, Hdaf 4.78–5.48%, Ndaf 0.7–1.3% (all percentages by weight). The results of the total sulfur (ISO 334:1992) of the lignite samples were from 0.59 to 4.49% by weight.
The above analyses confirm the low degree of coalification (low rank) of the studied lignite. It also indicates the low random reflectance of the euulminite present, which ranges from 0.26 to 0.29% depending on the depth (Table 2).
The Methane Sorption Results
Before the sorption experiment, all samples were hand-crushed, to measurement fraction of 0.5–1.5 mm. Next, approximately 50 g of the sample was placed in the measuring volume of the apparatus. As the specimens were prepared for the measurements, previously absorbed/adsorbed gases and vapors had to be removed from the coal surface. The measurement procedure was as follows: (a) degassing of the apparatus, with valves V1 and V2 open; (b) closure of valve V1 and filling of the reference cell; (c) feeding of the sorbate by opening the valve V1; and (d) when equilibrium is attained, closure of valve V1 and the repeated filling of the reference cell.
The criterion for determining whether equilibrium has been attained is the achievement of a time-constant value of gas pressure in the ampoule. Subsequent points of the isotherm are derived by dosing further quantities of gas. The quantity of the adsorbed gas is derived from the pressure difference before and after sorption, taking into account the dead volume of the measuring apparatus (obtained by deducting the volume of the coal grains from the volume of the whole apparatus). The difference between ideal and real gases is an important consideration when processing the experimental data. Within the range of the test pressures and temperatures, this difference could be precisely determined using the Beattie–Bridgeman equation (Çengel and Boles 2015). The molar gas volumes were determined for the given pressure and temperature values.
The sorption measurements show that for most of the samples, the methane sorption isotherms are of linear type (Fig. 4). The highest levels of sorption were obtained for samples 22, 57 and 33. These samples also exhibited the greatest increase in sorption in the initial pressure range.
In the vitrinite varieties of the bituminous coal, depending on rank, a high proportion of the total porosity is accounted for by the micro-pores (e.g., Baran et al. 2010; Czerw 2011; Teng et al. 2017). In the ortholignites, however, in spite of their often relatively high porosity (which for the Bełchatów sample reaches almost 5% (Macuda et al. 2011). The dominant pore types are meso- and macro-pores, with only a small quantity of micro-pores (Baran et al. 2013, 2014). This is confirmed by studies of ortholignite from four Polish deposits, carried out by the method of nitrogen adsorption at 77 K (Henc et al. 2018). The proportion of micro-pores (pores with a diameter of less than 2 nm, according to the IUPAC classification) in the studied lignites is in the range of 2–3% (Table 4 and Fig. 5). The dominant type is meso-pores (2–50 nm), which in the lignite from the Złoczew and Bełchatów deposits account for 50–66%, while a significant contribution also comes from the macro-pores (larger than 300 nm). This pore distribution determined for the structure of the studied lignite does not correlate with the specific adsorption surface area determined from BET isotherms, which is highest (6.32 m2/g) for the Złoczew lignite, somewhat lower (3.22 m2/g) for the Turów lignite, and low for the other studied deposits. This correlation does not explain the high (for the ortholignite) methane sorption capacity of the lignite from the Bełchatów deposit, which is similar on average to that of the Złoczew lignite. A consequence of such a pore distribution in the studied ortholignites will be the location of the methane molecules in the macro- and meso-porous structures, chiefly by way of compression of the gas in the elevated pressure conditions of the deposit, while the contribution of microporous adsorption will be much smaller than in the case of the bituminous coals. This is also confirmed by the approximately linear form of the sorption isotherms for the Złoczew lignite.
Pore distributions in the studied ortholignites (according Henc et al. 2018)
The experimental results, in accordance with the methodology currently applied in the sorption tests on the mined coal, were nonetheless described using a Langmuir adsorption isotherm:
where vm is a characteristic constant indicating the maximum monolayer capacity (dm3STP/kg), k is a constant (bar−1), and p is the equilibrium pressure (Table 5).
It is observed for the tested samples that the sorption capacity attains its highest values for samples with low real density, having an open structure that is fully accessible to methane. This is confirmed by the observed correlation between the real density and the monolayer capacity computed from the Langmuir adsorption isotherm (Table 6 and Fig. 6). As the sample density increases, an exponential drop in the sorption capacity is observed.
Assuming reversibility of sorption and desorption (Kudasik et al. 2017), based on the computed Langmuir constants, a calculation was also made of the quantity of methane that can be desorbed from a pressure of 10 bar to 1 bar and of the residual methane content Gr, which is the quantity of methane adsorbed at a pressure of 1 bar (Table 7). The largest desorption values were observed for samples 22, 33 and 57. The residual methane content was also high for these samples (Table 7). For all of these samples, the residual methane content was as high as 50% of the sorption capacity at the 10 bar. For the other samples, the corresponding value was around 30%.
The results of the adsorption tests were also described by means of a thermal sorption equation (Czepirski and Jagiełło 1989). This equation relies on the experimentally confirmed fact that the isosteric heat of adsorption remains practically invariant over a wide range of temperatures. For numerical computations, the equation was used in virial form:
where A0,…, An and B0,…, Bn are the parameters of best fit, n and k are the degrees of polynomials approximating the functions appearing in the equation, p is the equilibrium pressure, and v is the quantity of adsorbed gas. This equation may be treated as a regression equation giving the adsorbed quantity depending on the pressure and temperature.
The thermal sorption equation in special form enables the numerical calculation of the isosteric heat of adsorption as a function of the surface capacity:
The constant A0 from the equation enables the computation of the limiting value of the isosteric heat of adsorption as v → 0 (Table 8).
There are differences in the computed values of the limiting isosteric heat of adsorption. Higher values of the heat of adsorption at zero surface capacity would appear to indicate the presence of micro-pores. In the case of sample 22, which has the highest sorption capacity, the limiting isosteric heat of sorption is low. It may be concluded that this sample has a low proportion of micro-pores (as was confirmed in the BET test) and will favor the easier desorption of methane.
Comparisons with Other Polish Ortholignite Deposits
Apart from the Złoczew deposit, the mechanism of methane sorption has been previously investigated for four other ortholignite deposits in Poland: those of Bełchatów, Turów, Konin and Adamów (Macuda et al. 2011; Macuda and Zawisza 2007). Comparing the average sorption isotherms (Fig. 7), it is seen that these deposits can be divided into three clearly distinct groups:
-
those with the highest methane sorption rates, on average up to 1.7 dcm3/kg at a pressure of 10 bar (1 MPa); this group consists of the Bełchatów and Złoczew deposits;
-
those with lower methane sorption rates, reaching 0.8–0.9 dcm3/kg at 10 bar; this applies to lignite from the Turów and Adamów deposits;
-
lignite with the lowest methane sorption, from the Konin and Adamów deposits, where the total sorption was only 0.6 dcm3/kg.
The deposits in the first group (Bełchatów, Złoczew) have almost identical averaged isotherms of methane sorption, although there are large differences in values between individual samples: for example, values obtained in the Złoczew deposit range from 0.3 to 2.5 dm3/kg at a pressure of 0.9–1.0 MPa (Fig. 4).
What may be the reason for this? The ortholignites from the studied deposits are of the same age (continental Lower/Middle Miocene), and the degree of tectonic transformation is similar and small (local wide-radius folding with almost horizontal coal strata). There are, however, notable differences in the depth of the lignite found in these deposits. The Konin and Adamów lignite occurs at depths of less than 20 m, while in the deposits in the tectonic valleys (Bełchatów, Złoczew) the depths are much greater, ranging from 250 to 350 m. The Turów deposit is intermediate in this regard. There is a strong link between the depth of the lignite in Polish deposits and its petrographic composition, which varies chiefly in the degree of the gelification of the components from the huminite group: the deeper the coal, the higher is its degree of gelification—that is, the greater is its content of ulminite, gelinite and densinite. This variation is clearly reflected in the color of the coal (supported by the euulminite random reflectivity index), which becomes brown-black or black at greater depths. One of the symptoms of gelification in the case of such increasing coalification is exogenous cleavage, a dense system of cracks resulting from “drying out,” observed microscopically in the form of fissures with the dimensions of macro-pores and, it would appear, meso-pores. These provide routes for methane to reach the micro-pores, but they are also a place where it becomes compressed under increasing orogenic pressure.
It is claimed in the literature that macerals of the vitrinite group, also of the huminite group in coal with a lower degree of coalification, have the greatest effect on the sorption and desorption of methane (Crosdale et al. 1998; Chalmers and Marc Bustin 2007; Macuda et al. 2011; Keshavarz et al. 2017). With this in mind, an analysis was made of the effect of the principal macerals and the degree of gelification of the studied Złoczew lignite. Furthermore, the results were compared with the monolayer capacity v computed from the sorption isotherms.
To compute the gelification index, the proposal put forward by (George 1982) was selected as the simplest method, which also expresses directly the proportion of fully gelified macerals in the ortholignite. The formula is as follows:
The computed index GI has a directly proportional correlation with the monolayer sorption parameter vm, with a correlation coefficient r = 0.93. The correlation is statistically significant, and the coefficient of determination R2 = 0.86 indicates that only 14% of the results deviate from the determined regression equation (Fig. 8).
Referring to the interpretation of rates of methane sorption in the case of ortholignite samples from the Bełchatów deposit (Macuda et al. 2011) and with the aim of giving a more precise interpretation of the value GI as calculated from the above formula, an investigation was made of the correlations of the principal gelified macerals (ulminite, densinite) and the non-gelified macerals (attrinite) with the monolayer capacity vm (Kwiecińska and Wagner 1997). In the case of total densinite and ulminite, a statistically significant simple relationship was obtained with correlation coefficient r = 0.96 and coefficient of determination R2 = 0.96. The latter value indicates that only 4% of the results lie outside the confidence interval (α = 0.95) determined by the regression equation (Fig. 9). This shows that densinite and ulminite, the most numerous macerals of the huminite group in the studied lignite, are probably responsible for the rate of methane sorption.
Methane sorption is affected differently by the content of attrinite, the principal maceral of detritic and xylodetritic coal. Its petrographic formation, described as detritic, reveals chiefly the presence of macro-pores and meso-pores. Its correlation with the value of vm is high, but inversely proportional, with coefficients r = − 0.93 and R2 = 0.86 (Fig. 10). This petrographic component is thus found to reduce the quantity of methane sorption in ortholignite.
Conclusions
Ortholignite from the Złoczew deposit was found to have the greatest methane sorption capacity of any of the Polish deposits studied to date, comparable with lignite from the Bełchatów deposit. Both of these locations have the same genetic and morphological type (tectonic depressions) and lignite found at similar depths, ranging from 250 to 350 m.
The results of the sorption measurements on the Złoczew lignite deposit indicate linear sorption isotherms. The highest sorption capacity is found for the samples of gelified coal. These samples also exhibited the fastest increase in sorption in the initial pressure range.
Based on the computed values of the Langmuir constant, a determination was made of the quantity of methane that may be desorbed from a pressure of 10 bar to 1 bar and of the residual methane content Gr. Desorption values ranged from 0.81 to 1.79 dcm3/kg and correspond to the variable residual gas capacity (0.03–1.07 dcm3/kg). For all of the tested samples, the residual methane content is between 30 and 50% of the sorption capacity at a pressure of 10 bar.
The thermal sorption equations were also used to compute values of the limiting isosteric heat of adsorption, which differ between samples. Higher values of the heat of adsorption at zero surface capacity may indicate the presence of a small quantity of micro-pores. In the case of the samples with the highest sorption capacity, the limiting isosteric heats of adsorption are low. It may be concluded that these samples have low proportions of micro-pores, which will favor the easier desorption of methane.
These results are compared with the measurements of the porosity of ortholignite from the Polish deposits, carried out using the method of nitrogen adsorption at 77 K. The proportion of micro-pores in the studied lignites is 2–3%, while the dominant pore fraction is the meso-pores, which in lignite from the Złoczew and Bełchatów deposits account for 50–66% of the total pores, with a significant contribution also coming from macro-pores. It is therefore concluded, as noted in several previous scientific works, that the significant adsorption of methane in ortholignite occurs chiefly in meso-pores, because of the compression of the gas under increased orogenic pressure.
The low sorption capacity of the studied lignite compared with bituminous coal indicates in general that gelified macerals make a significant positive contribution to methane sorption, and also that an increasing proportion of detritic macerals (attrinite) and mineral substances markedly lowers the capacity of the coal to adsorb methane. This is evidenced by the strong correlations between sorption parameters and quantities of densinite, ulminite and attrinite. It was thus found that one of the main petrographic factors determining the rate of methane sorption in ortholignite is the degree of gelification of macerals of the huminite group.
The values and high variability of the sorption capacity of ortholignite show that careful consideration should be given to this parameter in the preliminary testing and mining of deposits, since as the cited examples have shown that methane adsorbed in ortholignite can create a serious risk of explosion.
References
Baran, P., Broś, M., & Nodzeński, A. (2010). Studies on CO2 sorption on hard coal in the near-critical area with regard to the aspect of sequestration. Archives of Mining Sciences, 55(1), 59–67.
Baran, P., Cygankiewicz, J., & Zarębska, K. (2013). Carbon dioxide sorption on polish ortholignite coal in low and elevated pressure. Journal of CO2 Utilization, 3–4, 44–48.
Baran, P., Zarebska, K., & Nodzeński, A. (2014). Energy aspects of CO2 sorption in the context of sequestration in coal deposits. Journal of Earth Science, 25(4), 719–726.
Borowicz, A., Frankowski, R., Gądek, A., Jończyk, W., Specylak-Skrzypecka, J., & Ślusarczyk, G. (2007). The lignite deposit Złoczew—Geological construction, resources and perspective of operation. Górnictwo i Geoinżynieria, 31(2), 141–150.
Çengel, Y., & Boles, M. (2015). Thermodynamics: An engineering approach (8th ed.). McGraw-Hill Companies.
Chalmers, G. R. L., & Marc Bustin, R. (2007). On the effects of petrographic composition on coalbed methane sorption. International Journal of Coal Geology, 69(4), 288–304.
Crosdale, P. J., Beamish, B. B., & Valix, M. (1998). Coalbed methane sorption related to coal composition. International Journal of Coal Geology, 35(1–4), 147–158.
Czepirski, L., & Jagiełło, J. (1989). Virial-type thermal equation of gas—Solid adsorption. Chemical Engineering Science, 44(4), 797–801.
Czerw, K. (2011). Methane and carbon dioxide sorption/desorption on bituminous coal—Experiments on cubicoid sample cut from the primal coal lump. International Journal of Coal Geology, 85(1), 72–77.
Czerw, K., Zarębska, K., Buczek, B., & Baran, P. (2016). Kinetic models assessment for swelling of coal induced by methane and carbon dioxide sorption. Adsorption. https://doi.org/10.1007/s10450-016-9775-z.
Flores, R. M. (2014). Chapter 4 - Coalification, gasification, and gas storage. In R. M. B. T.-C. & C. G. Flores (Eds.), Coal and coalbed gas. Fueling the future (pp. 167–233). Boston: Elsevier. https://doi.org/10.1016/B978-0-12-396972-9.00004-5.
Gensterblum, Y., van Hemert, P., Billemont, P., Battistutta, E., Busch, A., Krooss, B. M., et al. (2010). European inter-laboratory comparison of high pressure CO2 sorption isotherms II: Natural coals. International Journal of Coal Geology, 84(2), 115–124. https://doi.org/10.1016/J.COAL.2010.08.013.
George, A. (1982). Latrobe Valley brown coal-lithotypes: Macerals: Coal properties. Australian Coal Geology, 4(1), 111–130.
Gründger, F., Jiménez, N., Thielemann, T., Straaten, N., Lüders, T., Richnow, H.-H., et al. (2015). Microbial methane formation in deep aquifers of a coal-bearing sedimentary basin, Germany. Frontiers in Microbiology, 6, 200. https://doi.org/10.3389/fmicb.2015.00200.
Henc, M., Tomaszewicz, M., Tsuboi, Y., Sawicki, J., Tomaszewicz, G., & Zuwała, J. (2018). Use lignite in gasification—Review of Polish Wroclaw - Japan Project „UCESP”. In DRYLING project Final Workshop: Competitive pre-drying technologies and firing concepts for flexible and efficient lignite utilization (pp. 1–22). http://wme-z1.pwr.edu.pl/wp-content/uploads/2017/06/S33_Tomaszewicz_gasification_ICHPW.pdf.
Hycnar, E., Ratajczak, T., Jonczyk, W., & Wagner, M. (2015). Ekologiczne kryteria oceny jakości węgla brunatnego na przykładzie złoża Bełchatów. Zeszyty Naukowe Instytutu Gospodarki Surowcami Mineralnymi i Energią PAN, 91, 81–89.
Kasiński, J., Piwocki, M., Sadowska, E., & Ziembińska-Tworzydło, M. (2010). Lignite of the Polish Lowlands Miocene: characteristics on a base of selected profiles. Biuletyn Państwowego Instytutu Geologicznego, 439, 99–154.
Keshavarz, A., Sakurovs, R., Grigore, M., & Sayyafzadeh, M. (2017). Effect of maceral composition and coal rank on gas diffusion in Australian coals. International Journal of Coal Geology, 173, 65–75.
Kudasik, M., Skoczylas, N., & Pajdak, A. (2017). The repeatability of sorption processes occurring in the coal-methane system during multiple measurement series. Energies, 10(5), 4013–4033.
Kwiecińska, B., & Wagner, M. (1997). Classification of qualitative features of brown coal from Polish deposits according to petrographical, chemical and technological criteria. Kraków: Wydawnictwo Centrum PPGSMiE PAN.
Macuda, J., Nodzeński, A., Wagner, M., & Zawisza, L. (2011). Sorption of methane on lignite from Polish deposits. International Journal of Coal Geology, 87(1), 41–48. https://doi.org/10.1016/J.COAL.2011.04.010.
Macuda, J., & Zawisza, L. (2007). Occurrence of methane in lignite seams. Górnictwo i Geoinżynieria, 31(2), 445–452.
Mayumi, D., Mochimaru, H., Tamaki, H., Yamamoto, K., Yoshioka, H., Suzuki, Y., et al. (2016). Methane production from coal by a single methanogen. Science, 354(6309), 222–225.
Miller, B. G. (2005). The effect of coal usage on human health and the environment. Coal Energy Systems. https://doi.org/10.1016/B978-012497451-7/50003-6.
Orem, W. H., & Finkelman, R. B. (2003). Coal Formation and Geochemistry. In H. D. Holland & K. K. B. T.-T. on G. Turekian (Eds.), Treatise on geochemistry (pp. 191–222). Oxford: Pergamon. https://doi.org/10.1016/B0-08-043751-6/07097-3.
Peryt, T., & Piwocki, M. (2004). Paleogen i neogen w zapadliskach i rowach tektonicznych. In Budowa geologiczna Polski, t. I Stratygrafia, cz. 3a Kenozoik, Paleogen, Neogen (pp. 134–142). Warszawa: Państwowy Instytut Geologiczny - PIB.
Sakurovs, R., Day, S., & Weir, S. (2009). Causes and consequences of errors in determining sorption capacity of coals for carbon dioxide at high pressure. International Journal of Coal Geology, 77(1–2), 16–22. https://doi.org/10.1016/J.COAL.2008.07.001.
Sawicki, J. (2010). Hydrogeologiczne i górnicze uwarunkowania eksploatacji złoża węgla brunatnego “Złoczew”. Prace Naukowe Instytutu Górnictwa Politechniki Wrocławskiej. Studia i Materiały, 131(38), 127–148.
Stach, E., Chandra, D., Mackowsky, M., Taylor, G., Teichmüller, M., & Teichmüller, R. (1982). Stach’s Textbook of coal petrology. Stuttgart: Borntraeger.
Strąpoć, D., Mastalerz, M., Dawson, K., Macalady, J., Callaghan, A. V., Wawrik, B., et al. (2011). Biogeochemistry of microbial coal-bed methane. Annual Review of Earth and Planetary Sciences, 39(1), 617–656.
Teng, J., Mastalerz, M., & Hampton, L. (2017). Maceral controls on porosity characteristics of lithotypes of Pennsylvanian high volatile bituminous coal: Example from the Illinois Basin. International Journal of Coal Geology, 172, 80–94.
Wagner, M. (2007). Zmienność petrologiczno-sedymentologiczna i własności technologiczne kredy jeziornej w osadach neogenu typu wapiennego zapadliska tektonicznego na przykładzie złoża węgla brunatnego, “Szczerców”. Kraków: AGH Uczelniane Wydawnictwa Naukowo-Dydaktyczne. (in Polish, English abstract).
Widera, M. (2013). Changes of the lignite seam architecture—A case study from Polish lignite deposits. International Journal of Coal Geology, 114, 60–73.
Widera, M. (2016). Depositional environments of overbank sedimentation in the lignite-bearing Grey Clays Member: New evidence from Middle Miocene deposits of central Poland. Sedimentary Geology, 335, 150–165.
Acknowledgments
This study is supported as the AGH University of Science and Technology, Statutory Research Project 11.11.210.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macuda, J., Baran, P. & Wagner, M. Evaluation of the Presence of Methane in Złoczew Lignite: Comparison with Other Lignite Deposits in Poland. Nat Resour Res 29, 3841–3856 (2020). https://doi.org/10.1007/s11053-020-09691-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-020-09691-7