Abstract
This work presents attempts to synthesize silver-doped copper nitride nanostructures using chemical solution methods. Copper(II) nitrate and silver(I) nitrate were used as precursors and the oleylamine as a reducing and capping agent. Homogeneous Cu3N/Ag nanostructures with a diameter of ~ 20 nm were obtained in a one-pot synthesis by the addition of the copper(II) salt precursor to the already-synthesized silver nanoparticles (Ag NPs). Synthesis in a two-pot procedure performed by adding Ag NPs to the reaction medium of the Cu3N synthesis resulted in the formation of a Cu3N@Ag nanocomposite, in which Ag NPs are uniformly distributed in the Cu3N matrix. The morphology, structure, and chemical composition of the obtained specimens were studied by TEM, XRD, XPS, and FT-IR methods, while optical properties using UV–Vis spectroscopy and spectrofluorimetry. The band gap energy decreased for Cu3N/Ag (Eg = 2.1 eV), in relation to pure Cu3N (Eg = 2.4. eV), suggesting the insertion of Ag atoms into the Cu3N crystal lattice. Additionally, Cu3N and Cu3N/Ag nanostructures were loaded on graphene (GNP) and tested as a catalyst in the oxygen reduction reaction (ORR) by cyclic voltammetry (CV) and linear sweep voltammetry (LSV). The Cu3N/Ag-modified GNP hybrid material revealed catalytic activity superior to that of Cu3N-based GNP hybrid material and pure GNP, comparable to that of a commercial Pt/C electrode.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ternary transition metal nitrides (TTMNs) have attracted growing interest due to their structural diversity, various properties, applications, and relatively unexplored chemistry [1]. As a substrate for TTMNs synthesis, binary copper(I) nitride (Cu3N) can be used, which is known for its unique optoelectronic, catalytic, and electrochemical properties [2]. The specific cubic anti-ReO3 structure gives a possibility of Cu3N modification by inserting other atoms into the inner and/or outer crystal lattice, which imposes significant changes in copper nitride physicochemical properties [2]. There are numerous computational studies confirming that the properties of Cu3N can be varied by doping various transition metal atoms, e.g., Sc, Ti, V, Cr, Mn, Cu, Zn, Pd, and Ag [3,4,5,6,7,8,9,10]. However, while different physical and chemical synthesis methods for binary Cu3N are known, the synthesis of ternary copper nitrides Cu3TMxN (TM = transition metal) is limited to physical vapor deposition (PVD), such as magnetron sputtering. To the best of our knowledge, only palladium-doped Cu3N nanocrystals and gold-decorated Cu3N nanocrystals have been obtained using solution-based synthesis [11]. Specimens obtained using chemical synthesis methods and some of transition metal-doped Cu3N thin films obtained by PVD methods are listed in Table 1.
One of the most intriguing TM dopants is silver since it is isovalent to copper, and thus, it could retain similar interactions after replacing the Cu atom [19]. According to reports, both the insertion of the silver atom in the interstitial sites and the replacement of the copper atom with silver expand the crystal unit cell [8, 19]. This phenomenon can be related to the larger size of the silver atom (covalent radius 145 pm and Ag+ ionic radius 129 pm) compared to the copper atom (covalent radius 132 pm and Cu+ ionic radius 91 pm). However, the unit cell expansion is smaller in the case of interstitial doping, most probably due to retained cubic structure. In the case of substitutional doping, the cell expands along the z axis and transfers to tetragonal. Besides changes in the crystal structure, doping also affects electrical properties. Substituting Cu with Ag preserves the semiconducting nature with reducing band gap value. In contrast, the insertion of Ag into the vacant site induces semiconductor-to-metal transition [15, 19,20,21] of the bulk material. Considering the interesting predicted properties of silver-doped Cu3N structures, it seems worth developing new chemical synthesis routes for the mentioned materials.
In recent years, there has been observed an increased interest in the synthesis of Cu3N and Cu3N-based nanocrystals, using long-chain amines, such as oleylamine (OAm) or octadecylamine (ODA) [14, 22, 23], which act as high-boiling point solvents, reducing agents, and surfactants in a one-stage synthesis reaction [24]. It has been reported that by changing the synthesis reaction parameters, the morphology, size, and chemical composition of crystals can be varied in a controlled way [22, 24, 25]. When a mixture of precursors is used, complex bimetallic compounds, including ternary nitrides, can be obtained. A unique example implemented for Cu3N is nanostructured anti-perovskite Cu3PdN synthesized in a one-pot reaction of copper and palladium salts in an oleylamine/octadecene mixed solvent [11]. Oleylamine was also used to synthesize heterostructures of Cu3N nanocrystals decorated with gold nanoparticles [14].
Since the oleylamine (OAm) reagent has been successfully employed to synthesize colloidal zero-valent silver nanoparticles [26], one can presume that the synthesis of Cu3N nanocrystals should be compatible with the synthesis of silver NPs. Therefore, we hypothesize that it is possible to obtain homogenous Ag-doped Cu3N nanocrystals in the chemical synthesis using the oleylamine reagent. We suppose that changing the reaction conditions allows the composition and morphology of the resulting nanostructures to be controlled. This control may open new possibilities for applying copper nitride-based materials in innovative technologies.
Among the many potential applications of Cu3N-based materials, electro-catalytic performance has become an intensively studied research area. Binary copper nitride is considered to be a promising catalyst in electrochemical reduction reactions, including oxygen reduction reaction (ORR) [11, 23, 27, 28]. Currently, the main goal in the search for new ORR catalysts is to minimize the content of noble metals while maintaining satisfactory performance. Various composite and bimetallic nanostructured systems are being designed to achieve this goal. An example of Cu3N-based ternary nitride studied toward the ORR is Cu3PdN nanocrystals exhibiting greater activity than pure Cu3N and Pd nanocrystals [11]. In this work, the electrochemical properties of fabricated nitrides deposited onto graphene nanoplatelets (GNP) were investigated to show whether an enhancement of the electro-activity in the ORR could be achieved by modification of Cu3N with silver atoms.
In this study, we explore different approaches to synthesize silver-modified Cu3N nanomaterials using chemical synthesis routes for the first time. The electrocatalytic properties of the resulting structures were studied toward oxygen reduction reaction.
Materials and methods
Materials
Copper(II) nitrate trihydrate (Cu(NO3)2∙3H2O) (pro analysis (p.a.)) and n-hexane (99%) were purchased from CHEMPUR (Poland). Silver nitrate (AgNO3) (99 + %, ACS reagent) and 1-octadecene (90%) were supplied by Sigma-Aldrich, whereas oleylamine (80–90%) by Acros Organics. Ethanol (≥ 99.8%) was purchased from Honeywell, while toluene (p.a.) and chloroform from POCH (Poland). Pristine graphene nanoplatelets (GNPs) with a surface area of 750 m2/g were supplied by Sigma-Aldrich (900,407).
Synthesis of Cu3N nanocrystals
Copper nitride nanocrystals were fabricated via modified method described in literature. The procedure is presented in Supporting Information (SI).
Synthesis of Ag-modified Cu3N
The syntheses of Ag-modified Cu3N were conducted in different ways described below: (i) one-pot synthesis of Cu3N/Ag mixture, (ii) two-pot synthesis of Cu3N@Ag nanocomposite, and (iii) one-pot two-step synthesis of Cu3N/Ag nanostructures. The scheme of the synthesis procedures is presented in Fig. 1.
One-pot synthesis of Cu3N/Ag mixture
Cu(NO3)2∙3H2O, 60 mg (0.25 mmol), and AgNO3, 17 mg (0.1 mmol) were placed in a 50-ml reaction flask, followed by the addition of 5 ml OAm (15.2 mmol) and 5 ml ODE (15.6 mmol). The reaction mixture was then heated and purified analogously as for pure Cu3N (SI).
Synthesis of Cu3N@Ag nanocomposite
In the first step, Ag NPs were synthesized using AgNO3 precursor and OAm/ODE solvents in a 6-h procedure described in Supporting Information. Then, AgNP dispersion in toluene (~ 0.1 ml) was diluted to 3 ml, placed in a 50-ml round bottom flask, and left overnight for solvent evaporation. To the solid residue, 60 mg of Cu(NO3)2∙3H2O, 5 ml of ODE, and 5 ml of OAm were added, and the synthesis and purification processes were performed in an analogous way as for pure Cu3N nanocrystals (SI).
Synthesis of Cu3N/Ag nanostructures
In a typical synthesis, Ag NPs were obtained as described above using 14 mg (0.082 mmol) of AgNO3. When the suspension was cooled to 50 °C, 60 mg of Cu3N∙3H2O was added, and the reaction was conducted as for Cu3N. The obtained dark brown suspension was cooled down to room temperature. The solid precipitate was obtained by adding ethanol and centrifuged at 4000 rpm for 15 min. The precipitate was dispersed in n-hexane, precipitated by adding 80 ml of ethanol, and centrifuged at 4000 rpm for 5 min (repeated two times). The final product was dispersed in n-hexane and stored in a glove box filled with Ar.
Graphene-based hybrid material preparation
Dried Cu3N (22 mg) and Cu3N/Ag nanostructure (26.5 mg) pellets were dispersed in a 50 ml of CHCl3, mixed with 0.2 g of GNP powder, and filled with Ar gas. After 3 days, the mixture was dried under vacuum using Schlenk techniques. The resulting fine powder was further used as a catalyst for the ORR experiments.
Characterization of Cu3N and Ag-modified Cu3N materials
Surface morphology and composition were studied using transmission electron microscopy (TEM) with scanning transmission electron microscopy (STEM) mode, selected area electron diffraction (SAED), and energy-dispersive X-ray spectroscopy (EDX) with a Tecnai F20 X-Twin microscope (FEI Europe). The purified samples dispersed in a solvent (hexane or chloroform) were placed on nickel grids and allowed to evaporate. The X-ray powder diffraction (XRD) was conducted using an X’Pert Pro diffractometer (Malvern Panalytical Ltd.) with CuKα radiation. The Fourier-transform infrared (FT-IR) spectroscopy analysis of solid or liquid samples was carried out with an FT-IR Vertex 70 V spectrometer (Bruker Optik) using the attenuated total reflectance (ATR) mode in the spectral range of 100–4000 cm−1. The samples were washed several times and dried to the powder under vacuum before the XRD and FT-IR analyses. UV–Vis spectra of samples dispersed in chloroform were registered with a V-750 spectrometer (Jasco) using a bandwidth of 2 nm and a scan speed of 200 nm/min. Fluorescence spectra of samples dispersed in chloroform were collected with an FP-8200 spectrofluorometer (Jasco) using 5-nm excitation and emission bandwidth and a scan speed of 1000 nm/min. X-ray photoelectron spectroscopy (XPS) was used to investigate the surface composition and chemical state of the samples. The measurements were performed in ultra-high vacuum (UHV) conditions with a base pressure ≤ 2∙10−10 mbar. The excitation source was the Al Kα lamp (1486.6 eV). Photoelectron energy was recorded with the VG-Scienta R3000 hemispherical analyzer. The axis of the analyzer input slit coincided with the normal of the samples. Both survey and high-resolution spectra were recorded. In the latter case, the measuring step was set to 100 meV. Measurement raw data were deconvoluted to a Shirley background and Gauss-Lorentz shapes using the CasaXPS® software. For the majority of the sample components, standard line shapes, i.e., product of a Gaussian with a Lorentzian (70% of Gaussian and 30% of Lorentzian), were used. Only in the case of copper and silver, the Lorentzian percentage was higher - 80 and 72%, respectively.
Characterization of graphene-based hybrid materials
The structure of GNP hybrid materials was analyzed at −196 °C using an automatic adsorption instrument, ASAP 2010 (Micromeritics, Norcross, GA, USA). Before analysis, samples were outgassed in a vacuum at 105 °C for 24 h. The Brunauer–Emmett–Teller (BET) model calculated the surface area of obtained samples. Pore size was determined from the nonlocalized density functional theory (NLDFT) method. Additionally, the t-plot method was used to characterize the structure parameters of representative materials.
Electrochemical tests were carried out on the Autolab electrochemical analyzer (PGSTAT128N, the Netherlands). Additionally, the electrochemical activity of the produced hybrid systems was determined by means of a rotating disc electrode (RDE). Before starting the tests, the surface of glassy carbon (GC, 3 mm) was polished, and then, the appropriate amount of the catalyst with a packing of 0.4 mg cm−2 was applied and prepared in the following way: 2.5 mg of the resulting catalyst was dispersed in a liquid mixture of distilled water, ethanol, and Nafion (0.5 wt%) for 45 min. The activity of the obtained materials was checked on the basis of the measurements of linear voltammetry (LSV) and cyclic voltammetry (CV) in a three-electrode system, in which Ag/AgCl was used as the reference electrode, the platinum plate was the counter electrode, and the glassy carbon (GC, diameter 3 mm) with the applied catalyst as the working electrode [29]. The catalytic activity of all measured materials was compared to a platinum-based commercial carbon material (20 wt% of Pt, Sigma-Aldrich) as reference material [29]. Electrochemical measurements were performed according to the procedure in our previous works [29, 30].
Results and discussion
One-pot synthesis of Ag nanoparticles modified with Cu3N resulted in the formation of two well-separated components, i.e., polyhedral Ag microcrystals (200–300-nm length) covered with a 25-nm-long shell of Cu3N nanocrystals (Fig. S1). Crystals were poorly fused, which could be caused by the significant size differences between Ag nanocrystals and Cu3N (ca. 8:1). In the following part of the article, results for two much more uniform types of materials, i.e., Cu3N@Ag nanocomposite and Cu3N/Ag nanostructures, are presented.
Characterization of Cu3N@Ag nanocomposite
The literature study indicates that the size of Ag crystals depends on the aging and ripening process. Using a lower temperature (150 °C) and a longer time (5–8 h), the reaction results in a reduced size of Ag crystals [26]. In the case of Cu3N, synthesis requires higher temperatures (230–260 °C) and a shorter reaction time (10–20 min). The synthesis was modified and carried out in a two-step route to prepare a nanomaterial containing both species with comparable sizes. In particular, AgNPs were synthesized, purified, and finally added to the reagent mixture of Cu3N synthesis. TEM images of obtained spherical Ag nanoparticles with a diameter of 10–15 nm are presented in Supporting Information (Fig. S2). TEM images of pure Cu3N and Ag-modified Cu3N are presented in Fig. 2. The Cu3N nanocrystals resemble distorted cubes of 20–30 nm in length (Fig. 2aa). In the case of Ag-modified Cu3N, a composite material was obtained, consisting of Ag NPs covered with a shell of Cu3N nanoparticles (10–15 nm) (Fig. 2c, d). The d-spacings determined from the lattice fringes correspond well to the characteristic (100) and (110) planes of Cu3N (d = 0.381 and 0.265 nm, respectively) and (200) and (111) planes of Ag (d = 0.205 and 0.234 nm, respectively) [31]. The outer layer of the nanocomposite was formed out of the organic film, indicating the presence of the long-chain capping agent. The sample will be hereinafter referred to as Cu3N@Ag nanocomposite.
The XRD results (Fig. 3a) confirmed the heterostructure of the obtained nanomaterial. The XRD pattern of Cu3N reveals reflections characteristic of the anti-ReO3 structure of copper nitride (23.48°, 33.40°, 41.20°, 47.93°, 53.98°, 56.65°, 70.03°, 74.92°, 80.21°, 84.66°, 89.25°), without any impurities. The diffractogram for Cu3N@Ag composite, besides the Cu3N peaks, indicates reflections at 38.24°, 44.27°, 64.56°, 77.59°, and 81.73°, corresponding to the fcc structure of Ag crystals. In addition, besides the Cu3N and Ag lines, three low-intensity peaks at 43.39°, 50.33°, and 74.18° can be assigned to metallic Cu. This may indicate that the addition of silver induced the decomposition of Cu3N to metallic Cu.
The FT-IR spectra of Cu3N@Ag composite, pure Cu3N nanocrystals, and free OAm are shown in Fig. 3b. The bands in the OAm spectrum are also observed in the spectra of Cu3N and Cu3N@Ag composite, indicating the presence of the organic capping ligand. The bands at 2916 and 2848 cm−1 are assigned to C–H stretching vibrations in the methylene group, whereas bands at 1452 cm−1 and 1375 cm −1 correspond to C–H bending vibrations from the methyl group, and the band at 965 cm−1 can be assigned to C=C–H bending modes. The amine bands at 1545 cm−1 correspond to the N–H bending vibrations. The bands at 790 and 720 cm−1 are present only in the OAm spectrum and can be assigned to the N–H wagging vibrations. In the spectra of Cu3N and Cu3N@Ag, new band was noted at 2125 cm−1, which can be assigned to the C≡N stretching vibrations due to the formation of a nitrile bond. The latter was confirmed by NMR spectra by other authors [25]. There is also a low-intensity band at 1730 cm−1, which can be assigned to C=O stretching vibrations indicating the presence of some impurities, e.g., due to the formation of carbonyl groups or amide species [32]. The spectrum of pure Cu3N exhibits a strong band at 649 cm−1, which is characteristic for Cu–N valence stretching vibrations, whereas for Cu3N@Ag composite, the band is slightly shifted to 641 cm−1. The shift towards lower frequencies (8 cm−1) can be caused by the elongation of Cu–N distance due to the contact with silver nanoparticles in the composite.
To confirm the structure of Cu3N@Ag nanocomposite, X-ray photoelectron spectroscopy measurements were performed. The organic ligand components, i.e., carbon and oxygen, dominate in the XPS spectra. The signal from Cu3N and silver is also present, but the intensity of these components is low. The atomic surface concentration of Cu does not exceed 5%. In the case of Ag, it is only tenths of a percent. Such a low intensity does not allow for a reliable analysis of the CuLMM level. Given a large amount of long-chain capping agent on the particle surface, such results are not surprising, and similar intensities were noticed by other authors [33]. Furthermore, copper nitride is known to readily adsorb oxygen from atmospheric air, resulting in the carbon and oxygen content at even over 50% [34]. The XPS spectra of Cu 2p, Ag 3d, and N 1s levels are presented in Fig. 4. The Cu 2p3/2 peak was fitted with three peaks at 932.2, 931.2, and 929.2 eV. The strongest peak originates from the Cu atom bond with nitrogen in Cu3N, whereas other peaks indicate the coordination of Cu atoms with the organic ligand. The N 1 s spectra indicate three components related to N atoms from Cu3N and the complex structure of the organic ligand on the crystal’s surface. Attempting to adjust the Ag 3d level suggests the presence of one component derived from silver. The resulting spectrum reveals asymmetrical 3d5/2 and 3d3/2 peaks split by about 6 eV, characteristic of zerovalent Ag. The results are in good agreement with TEM images, confirming the composite nature of the obtained material.
Characterization of Cu3N/Ag nanostructures
To better control the silver content in the sample, a one-pot two-step synthesis has been examined. In this procedure, the first step was Ag NPs formation, followed by the addition of copper nitrate to the reaction mixture at the end of the Ag ripening process (all in one pot). The reaction was further performed analogously for the synthesis of pure Cu3N nanocrystals. The copper and silver reagent ratio in the mixture corresponded to the Cu/Ag atomic ratio of 3:1 (sample A1). TEM images (Fig. 5a, b) indicate formation of the uniform one-phase nanoparticles of diameter varying from 10 to 20 nm. The EDX quantification measurements indicated the presence of the long-chain capping agent bound on the crystal surface (85.0 at% carbon). However, when a single crystal was analyzed (Fig. 5c, d), both copper and silver were detected at 3.1 and 3.5 at%, respectively. The latter suggests the incorporation of silver atoms into the structure of Cu3N. The sample will be hereinafter referred to as Cu3N/Ag nanostructures.
Substoichiometric samples with Cu/Ag ratios of 6:1 (sample A2) and 30:1 (sample A3) were also synthesized to study the effect of silver content on the material morphology and composition. The TEM images of A2 and A3 samples (Fig. S3) reveal the particle erosion process started and the separation of Ag and Cu3N phases, resulting in the material similar to Cu3N@Ag nanocomposite. Reflections at 33.24°, 53.88°, and 70.10° (Fig. S4), corresponding to the (110), (210), and (220) Cu3N planes, respectively, exhibit the highest intensity for the A3 sample with a Cu/Ag ratio of 30:1. Analogously, the Ag peaks reveal the highest intensity for the A1 sample with a Cu/Ag ratio of 3:1. XRD patterns clearly indicate that the increase of Cu3N peak intensity is related to the silver content decrease. The diffractogram for A1 sample significantly differs from that for the Cu3N/Ag mixture obtained in one-pot synthesis, in which a similar amount of Ag+ precursor was added. For the Cu3N/Ag mixture, sharp peaks for Cu3N are observed, whereas for the Cu3N/Ag, these peaks have low intensity.
FT-IR spectra of samples modified with different content of Ag (Fig. S4) are similar to the Cu3N and Cu3N@Ag spectra; however, the organic ligand seems to have a more complex composition. The C=O vibration band at 1730 cm−1 gets higher intensity together with increasing the silver content in the sample. The Cu–N stretching vibration band appears at 638 cm−1 for the samples with Cu/Ag ratios of 30:1 and 6:1. The low-frequency shift of the valence band is caused by the elongation of the Cu–N distance due to the contact with silver and crystal lattice distortion.
The XPS spectra for Cu3N/Ag nanostructures (Fig. 6) are in agreement with the above-described spectra for Cu3N@Ag nanocomposite. The low intensity of signals resulted in one single Cu 2p3/2 peak at 932.1 eV, and the N 1s spectrum indicates one component. The Ag spectrum reveals asymmetrical 3d5/2 and 3d3/2 peaks split by 6 eV, which confirms the existence of Ag as a metal phase. Compared with the XRD and EDX results, it can be assumed that silver was introduced into the center of the Cu3N unit cell as a nanocluster. In other works related to doping the Cu3N structure with Ag or Pd atoms, the doping atom was also present as a metallic phase [11, 15].
Since it can be assumed that Ag was inserted into the Cu3N structure, it was justified to study the optical properties and to determine the band gap energy of Cu3N and Cu3N/Ag nanostructures (sample A1). For this purpose, UV–Vis and fluorescence spectroscopy measurements were performed using colloidal solutions of the nanocrystals in chloroform. For both Cu3N and Cu3N/Ag, two absorption bands can be observed: a sharp peak with a maximum at 290 nm and a broad peak at 430 nm (Fig. 7a). Until recently, only the band in the visible region was studied in the literature [22]. However, according to the latest reports, two absorption bands are typical for the colloidal Cu3N nanocrystals synthesized in long-chain amines [25]. The reported studies of the formation mechanism of Cu3N NCs indicated that as the time of the reaction increased, red shifting of the band in the visible region was observed [25]. When the reaction time was 60 min, decomposition of copper nitride to copper nanoparticles was noted, and the band was observed at about 600 nm [25]. Here, instead, we observe a significant increase in the intensity of this absorption band for the Cu3N/Ag nanostructures. It is known that the surface plasmon resonance band of silver nanoparticles, depending on the synthesis conditions and size of the particles, occurs in a similar region [26, 35, 36]. Therefore, it can be assumed that the addition of silver causes such an increase in intensity. While for pure Cu3N nanocrystals, the band at 430 nm was observed at each repeated measurement, regardless of the conditions and sample storage time, but in the case of Cu3N/Ag sample, this band disappeared with long storage time.
The band gap energies of direct and indirect transitions for the Cu3N and Cu3N/Ag (A1) samples, determined using the Tauc method (Fig. S5), are listed in Table 2. Reported band gap values for Cu3N range from 0.8 to about 2 eV, depending on the nature of synthesized material and the type of studied transition [2, 37]. Higher energies (over 3.6 eV) have also been reported for the transitions in the ultraviolet region [25]. Here, the band gaps for direct and indirect transitions in the UV region increase after the addition of silver. On the other hand, in the visible region, the Eg values decrease after silver doping. The narrowing of band gap energy of this transition has been observed in computational and some experimental reports and was explained by substituting the Cu atom with Ag. On the other hand, insertion of Ag into the body center of Cu3N structure resulted in semiconductor to metal transition [15, 20]. Taking into account that there are no other reports regarding the chemical synthesis of such structures, the conclusions about the type of Ag doping are relatively not certain.
For fluorescence measurements, the sample solutions were diluted until the absorbance was about 0.1–0.15. The emission spectra were measured using excitation wavelengths ranging from 250 to 400 nm. The highest emission intensity (almost the maximum detectable emission—10,000 arbitrary units) was observed for λex = 275 nm. A sharp fluorescence band with a maximum at 337 nm was detected for both samples (Fig. 7b). The emission maxima are red-shifted by almost 50 nm concerning the absorption maxima of the first transition. The emission spectrum for colloidal Cu3N has been reported in the range from 450 to 500 nm with a relatively low intensity (not exceeding 600 arbitrary units) [25, 33]. In the spectrum of Cu3N/Ag nanocrystals, an additional band at 500 nm is observed.
Graphene-based hybrid material analysis
Based on the conducted experiments and structural analysis of the obtained compounds, Cu3N/Ag (A1) nanostructures were selected for further research. Synthesis of the Cu3N/Ag nanostructures allowed for introducing an exact amount of silver into the material, in contrast to the Cu3N@Ag nanocomposite. Moreover, the obtained single-phase Cu3N/Ag nanostructures suggest the assumed incorporation of Ag into the Cu3N structure. Cu3N/Ag nanostructures and, for comparison, pure Cu3N nanocrystals were loaded on graphene platelets (GNPs). TEM images of the fabricated materials (Fig. 8) showed that both Cu3N/Ag and Cu3N nanostructures have been uniformly adsorbed on the graphene layers and do not exist except for the carbon matrix.
The nitrogen adsorption–desorption isotherms of representative samples are demonstrated in Fig. S6. According to the IUPAC classification, all nitrogen adsorption–desorption isotherms for the obtained materials are type II. Additionally, there are hysteresis loops that indicate the presence of mesopores [38]. Table 3 presents information on the structural parameters of the obtained materials. The results of the t-plot method confirm the presence of mesopores in the structure of the obtained materials. The contribution of the mesopore volume Vme to the total pore volume Vt increased from 87 to 97% in the hybrid systems. Moreover, there is a noticeable reduction in the surface area of the materials after the production of hybrid systems due to the adsorption effect. The tendency to decrease the surface area from 750 to 617 m2/g in the case of the GNP_Cu3N sample and 556 m2/g in the case of the GNP_Cu3N/Ag is correct and indicates the adsorption of Cu and Ag on the carbon surface and penetration into the pores of the material structure.
Electrocatalytic activity of graphene-based hybrid materials
Additionally, the catalytic activity of carbon-based hybrid materials was studied using cyclic voltammetry (CV) and linear sweep voltammetry (LSV) techniques. All electrochemical measurements were made in 0.1 M KOH saturated with oxygen and nitrogen. Cyclic voltammetry (CV) results were recorded at a 10 mV s−1 scan rate and in the potential range from 0 to 0.8 V, while linear sweep voltammetry (LSV) data was recorded at a 5 mV s−1 scan rate and a rotating disk electrode (RDE) speed in the range from 800 to 2800 rpm. The number of electrons (n) taking part in the oxygen reduction reaction was calculated on the basis of the LSV plot using the Koutecky-Levich (K-L) formulas [29, 30].
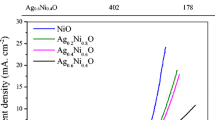
The voltammogram in Fig. 9a shows the CV curves, and the clear cathode peak (Ep) is visible in all hybrid systems. The value of the cathode peak for commercial platinum-based carbon (Pt/C, 20 wt%) is 0.76 V versus RHE, while for the obtained samples, clear peaks are observed in the range of 0.77–0.81 V versus RHE, respectively. Figure 9b presents LSV curves measured at a scan rate of 5 mV s−1 and an average rotation rate of 1600 rpm in an O2-saturated 0.1 M KOH solution. The shape of the curve of the GNP-Cu3N/Ag sample is the closest to that of a commercial Pt/C electrode. The results presented in Fig. 9c showed a linear character, which indicates the correct spread of electron transfer. Fundamentally, by determining the slope of the K-L plot, the number of electrons transferred in the ORR was estimated (Fig. 9d).
a CV curves of the obtained electrocatalysts and Pt/C catalysts in an O2-saturated 0.1 M KOH solution. b LSV curves of GNP_raw, GNP_Cu3N, GNP_Cu3N/Ag, and Pt/C catalysts measured at a scan rate of 5 mV s−1 and an average rotation rate of 1600 rpm in O2-saturated 0.1 M KOH solution. c Koutecky-Levich plots in the potential 0.5 V. d The number of transfer electrons in the oxygen reduction reaction
The ORR performance parameters are necessary to fully characterize the catalytic activity of the electrocatalyst and are listed in Table 4. The critical parameter “n” increased from 2.56 for the GNP_raw sample to 3.16 for the GNP_Cu3N material after the adsorption process. Moreover, the highest value of the number of electrons transferred was noticed for the GNP_Cu3N/Ag sample (n = 3.59). The onset potential (Eonset) for the ORR is an important criterion in evaluating the activity of an electrocatalyst. The results show that GNP_Cu3N/Ag sample has the closest Eonset to Pt/C catalyst.
Generally, electrochemical properties of carbon systems are affected by their porous structure, type of modifications, or the presence of heteroatoms such as sulfur, nitrogen, phosphor, or boron [29, 30]. Experimental measurements and DFT calculations indicate that Ag and Cu activities for ORR were located near Pt in a volcano plot [39, 40]. Here, the fabrication of a novel catalyst in which silver-modified Cu3N nanostructures were adsorbed on graphene nanoplatelets is demonstrated to improve the catalytic properties of raw graphene. Moreover, the insertion of silver into the copper nitride structure enhances the ORR performance.
The oxygen reduction reaction has been studied intensively and its mechanism described in many papers [29, 39, 41]. In general, the ORR process can proceed through a one-step four-electron pathway resulting in a complete reduction of oxygen to water [42]:
or through a two-step two-electron pathway, where oxygen is partially reduced to hydrogen peroxide:
An efficient catalyst for oxygen reduction reaction should favor the 4e− ORR pathway, similar to commercial electrodes. For metal-free catalysts, such as graphene-based materials, the ORR electron transfer number is usually about 2 [30, 43]. Conversely, according to the latest computational studies, Cu3N surface structure reveals high selectivity for the 4e− ORR process [44]. In addition, it was reported that coupling Cu3N nanostructures with carbon material enhances the ORR catalytic activity, compared to pure Cu3N and carbon, due to the possible synergistic effect [28, 45]. Therefore, the increase in the electron number from 2.59 to 3.16 after adding Cu3N nanostructures to the graphene nanoplatelets is reasonable. Further increase of the n value to 3.59 for the Cu3N/Ag structure indicates a four-electron ORR pathway as in the case of a commercial Pt/C electrode. Such a phenomenon suggests improving the synergistic effect of Cu and Ag atoms in the ternary intermetallic compound. Enhancing the electro-catalytic activity with the addition of noble metal was so far reported for Pd-doped Cu3N nanostructures [11]. Moreover, the ORR activity results correspond to the measured structural parameters of the samples, which showed a decrease in the surface area from the raw GNP sample through the GNP_Cu3N sample to the GNP_Cu3N/Ag sample. This suggests that the adsorption of ternary Cu3N/Ag nanostructures into the graphene pores affects the activity of the catalyst.
Considering the promising properties of binary copper nitride for electrochemical applications, such as ORR, CO2 reduction reaction (CO2RR), Li-ion batteries, and supercapacitors, it is justified to design new ternary copper nitride-based materials. Obtained Ag-modified Cu3N nanostructures have great potential for further electrochemical studies toward, e.g., battery production, LEDs, photovoltaic cells, and supercapacitors.
Conclusions
Different types of Cu3N nanocrystals modified with silver were synthesized using Cu(NO3)2∙3H2O and AgNO3 precursors and oleylamine reagent. Direct one-step synthesis, analogous to the reported synthesis of Cu3PdN nanocrystals, is not possible due to the required aging process for Ag NPs. The addition of silver nanoparticles to the reaction mixture for Cu3N synthesis resulted in the formation of Cu3N@Ag nanocomposite material. On the other hand, by adding Cu2+ precursor at the end of Ag NP synthesis, homogenous Cu3N/Ag nanocrystals were formed. One can suggest that insertion of Ag atoms into the Cu3N crystal lattice is possible in a controlled way. However, a certain amount of capping agent on the nanocrystals surface caused obstacles to the precision analysis of the obtained structure by XPS and EDX analysis. To the best of our knowledge, this is the first work regarding the chemical synthesis of silver-modified Cu3N nanomaterials. Analysis of the optical properties of the obtained materials indicated the bang gap narrowing effect as a result of the insertion of Ag atoms, which is consistent with the reported computational studies. The fabricated graphene-based Cu3N/Ag catalyst can be successfully used as electrode material in different devices using the oxygen reduction reaction. Due to its exceptional electrochemical performance, comparable to commercial Pt/C electrode, and controlled synthesis process, the proposed hybrid material should be a promising low-cost catalyst.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Moreover, the raw data are available from the corresponding author (RSz) upon reasonable request.
References
Tareen AK, Priyanga GS, Behara S et al (2019) Mixed ternary transition metal nitrides: a comprehensive review of synthesis, electronic structure, and properties of engineering relevance. Prog Solid State Chem 53:1–26. https://doi.org/10.1016/j.progsolidstchem.2018.11.001
Ścigała A, Szłyk E, Dobrzańska L et al (2021) From binary to multinary copper based nitrides—unlocking the potential of new applications. Coord Chem Rev 436:213791. https://doi.org/10.1016/j.ccr.2021.213791
Gao L, Ji AL, Zhang WB, Cao ZX (2011) Insertion of Zn atoms into Cu3N lattice: structural distortion and modification of electronic properties. J Cryst Growth 321:157–161. https://doi.org/10.1016/j.jcrysgro.2011.02.030
Zhao Y, Zhang Q, Huang S et al (2016) Effect of magnetic transition metal (TM = V, Cr, and Mn) dopant on characteristics of copper nitride. J Supercond Nov Magn 29:2351–2357. https://doi.org/10.1007/s10948-016-3511-5
Yu A, Ma Y, Chen A et al (2017) Thermal stability and optical properties of Sc-doped copper nitride films. Vacuum 141:243–248. https://doi.org/10.1016/j.vacuum.2017.04.024
Ji AL, Lu NP, Gao L et al (2013) Electrical properties and thermal stability of Pd-doped copper nitride films. J Appl Phys 113:043705. https://doi.org/10.1063/1.4788905
Fan XY, Wu ZG, Zhang GA et al (2007) Ti-doped copper nitride films deposited by cylindrical magnetron sputtering. J Alloys Compd 440:254–258. https://doi.org/10.1016/j.jallcom.2006.09.006
Pierson JF, Horwat D (2008) Addition of silver in copper nitride films deposited by reactive magnetron sputtering. Scr Mater 58:568–570. https://doi.org/10.1016/j.scriptamat.2007.11.016
Sun W, Bartel CJ, Arca E et al (2019) A map of the inorganic ternary metal nitrides. Nat Mater 18:732–739. https://doi.org/10.1038/s41563-019-0396-2
Cui XY, Soon A, Phillips AE et al (2012) First principles study of 3d transition metal doped Cu3N. J Magn Magn Mater 324:3138–3143. https://doi.org/10.1016/j.jmmm.2012.05.021
Vaughn DD, Araujo J, Meduri P et al (2014) Solution synthesis of Cu3PdN nanocrystals as ternary metal nitride electrocatalysts for the oxygen reduction reaction. Chem Mater 26:6226–6232. https://doi.org/10.1021/cm5029723
Jia J, Shao M, Wang G et al (2016) Cu3PdN nanocrystals electrocatalyst for formic acid oxidation. Electrochem commun 71:61–64. https://doi.org/10.1016/j.elecom.2016.08.009
Jia J, Hao X, Chang Y et al (2021) Rational design of Cu3PdN nanocrystals for selective electroreduction of carbon dioxide to formic acid. J Colloid Interface Sci 586:491–497. https://doi.org/10.1016/j.jcis.2020.10.112
Barman D, Paul S, Ghosh S, De SK (2019) Cu3N nanocrystals decorated with Au nanoparticles for photocatalytic degradation of organic dyes. ACS Appl Nano Mater 2:5009–5019. https://doi.org/10.1021/acsanm.9b00943
Xiao J, Qi M, Gong C et al (2018) Crystal structure and optical properties of silver-doped copper nitride films (Cu3N:Ag) prepared by magnetron sputtering. J Phys D Appl Phys 51:055305. https://doi.org/10.1088/1361-6463/aaa478
Lindahl E, Ottosson M, Carlsson JO (2018) Doping of metastable Cu3N at different Ni concentrations: Growth, crystallographic sites and resistivity. Thin Solid Films 647:1–8. https://doi.org/10.1016/j.tsf.2017.12.010
Fan XY, Li ZJ, Meng AL et al (2014) Study on the structure, morphology and properties of Fe-doped Cu3N films. J Phys D Appl Phys 47:185304. https://doi.org/10.1088/0022-3727/47/18/185304
Yu A, Hu R, Liu W et al (2018) Preparation and characterization of Mn doped copper nitride films with high photocurrent response. Curr Appl Phys 18:1306–1312. https://doi.org/10.1016/j.cap.2018.07.010
Sahoo G (2021) Manipulating elastic and mechanical properties of Cu3N through site selective Ag doping: first principles investigation. Mat Today Commun 29:102950. https://doi.org/10.1016/j.mtcomm.2021.102950
Sahoo G (2021) Site selective Ag doping in Cu3N and its consequences on structural and electronic properties A DFT study. Phys B Condens Matter 619:413238. https://doi.org/10.1016/j.physb.2021.413238
Lu N, Ji A, Cao Z (2013) Nearly constant electrical resistance over large temperature range in Cu3NMx (M = Cu, Ag, Au) compounds. Sci Rep 3:1–6. https://doi.org/10.1038/srep03090
Wu H, Chen W (2011) Copper nitride nanocubes: size-controlled synthesis and application as cathode catalyst in alkaline fuel cells. J Am Chem Soc 133:15236–15239. https://doi.org/10.1021/ja204748u
Yin Z, Yu C, Zhao Z et al (2019) Cu3N nanocubes for selective electrochemical reduction of CO2 to ethylene. Nano Lett 19:8658–8663. https://doi.org/10.1021/acs.nanolett.9b03324
Mourdikoudis S, Liz-Marzán LM (2013) Oleylamine in nanoparticle synthesis. Chem Mater 25:1465–1476. https://doi.org/10.1021/cm4000476
Kadzutu-Sithole R, Machogo-Phao LFE, Kolokoto T et al (2020) Elucidating the effect of precursor decomposition time on the structural and optical properties of copper(I) nitride nanocubes. RSC Adv 10:34231–34246. https://doi.org/10.1039/C9RA09546B
Chen M, Feng YG, Wang X et al (2007) Silver nanoparticles capped by oleylamine: formation, growth, and self-organization. Langmuir 23:5296–5304. https://doi.org/10.1021/la700553d
Liang ZQ, Zhuang TT, Seifitokaldani A et al (2018) Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat Commun 9:3828. https://doi.org/10.1038/s41467-018-06311-0
Mondal S, Raj CR (2018) Copper nitride nanostructure for the electrocatalytic reduction of oxygen: kinetics and reaction pathway. J Phys Chem C 122:18468–18475. https://doi.org/10.1021/acs.jpcc.8b03840
Kamedulski P, Skorupska M, Binkowski P et al (2021) High surface area micro-mesoporous graphene for electrochemical applications. Sci Rep 11:1–12. https://doi.org/10.1038/s41598-021-01154-0
Kamedulski P, Lukaszewicz JP, Witczak L et al (2021) The importance of structural factors for the electrochemical performance of graphene/carbon nanotube/melamine powders towards the catalytic activity of oxygen reduction reaction. Materials 14:2448. https://doi.org/10.3390/ma14092448
Chen R, Nuhfer NT, Moussa L et al (2008) Silver sulfide nanoparticle assembly obtained by reacting an assembled silver nanoparticle template with hydrogen sulfide gas. Nanotechnology 19:455604–455615. https://doi.org/10.1088/0957-4484/19/45/455604
Pesesse A, Carenco S (2021) Influence of the copper precursor on the catalytic transformation of oleylamine during Cu nanoparticle synthesis. Catal Sci Technol 11:5310–5320. https://doi.org/10.1039/D1CY00639H
Sithole RK, MacHogo LFE, Airo MA et al (2018) Synthesis and characterization of Cu3N nanoparticles using pyrrole-2-carbaldpropyliminato Cu(II) complex and Cu(NO3)2 as single-source precursors: the search for an ideal precursor. New J Chem 42:3042–3049. https://doi.org/10.1039/C7NJ05181F
Majumdar A, Drache S, Wulff H et al (2017) Strain effects by surface oxidation of Cu3N thin films deposited by DC magnetron sputtering. Coatings 7:64. https://doi.org/10.3390/coatings7050064
Vorobyova SA, Lesnikovich AI, Sobal NS (1999) Preparation of silver nanoparticles by interphase reduction. Colloids Surf A Physicochem Eng Asp 152:375–379. https://doi.org/10.1016/S0927-7757(98)00861-9
Bhui DK, Bar H, Sarkar P et al (2009) Synthesis and UV–vis spectroscopic study of silver nanoparticles in aqueous SDS solution. J Mol Liq 145:33–37. https://doi.org/10.1016/j.molliq.2008.11.014
Scigala A, Szłyk E, Rerek T et al (2021) Copper nitride nanowire arrays—comparison of synthetic approaches. Materials 14:603. https://doi.org/10.3390/ma14030603
Sotomayor FJ, Cychosz KA, Thommes M (2018) Characterization of micro/mesoporous materials by physisorption: concepts and case studies. Acc Mater Surf Res 3:34–50
Nørskov JK, Rossmeisl J, Logadottir A et al (2004) Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B 108:17886–17892. https://doi.org/10.1021/jp047349j
Thanh TD, Chuong ND, van Hien H et al (2018) CuAg@Ag core-shell nanostructure encapsulated by N-doped graphene as a high-performance catalyst for oxygen reduction reaction. ACS Appl Mater Interfaces 10:4672–4681. https://doi.org/10.1021/acsami.7b16294
Ma R, Lin G, Zhou Y et al (2019) A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. Npj Comput Mater 5:78. https://doi.org/10.1038/s41524-019-0210-3
Woo J, Lim JS, Kim JH, Joo SH (2021) Heteroatom-doped carbon-based oxygen reduction electrocatalysts with tailored four-electron and two-electron selectivity. Chem Commun 57:7350–7361. https://doi.org/10.1039/D1CC02667D
Kim HW, Bukas VJ, Park H et al (2020) Mechanisms of two-electron and four-electron electrochemical oxygen reduction reactions at nitrogen-doped reduced graphene oxide. ACS Catal 10:852–863. https://doi.org/10.1021/acscatal.9b04106
Wang J, Liu Z, Dai Z et al (2022) Multifunctional catalytic activity of Cu3N (001) surface: a first-principles study. ChemPhysMater. https://doi.org/10.1016/j.chphma.2022.10.001
Su CY, Liu BH, Lin TJ et al (2015) Carbon nanotube-supported Cu3N nanocrystals as a highly active catalyst for oxygen reduction reaction. J Mater Chem A 3(18983):18990. https://doi.org/10.1039/C5TA04383B
Acknowledgements
The authors would like to thank IDUB, OptoFoto, Torun grant title “Experimental and theoretical studies of nanostructured composite photosensitive layers” 2021/22.
Funding
This research work was financially supported by the National Science Centre of Poland (grant no. 2021/41/N/ST5/04413).
Author information
Authors and Affiliations
Contributions
Conceptualization: Aleksandra Ścigała, Robert Szczęsny, and Edward Szłyk. Material preparation, data collection, and analysis: Aleksandra Ścigała, Piotr Kamedulski, Marek Trzcinski, and Robert Szczęsny. Writing—original draft preparation: Aleksandra Ścigała and Piotr Kamedulski. Writing—review and editing: Robert Szczęsny and Edward Szłyk. Supervision: Robert Szczęsny and Edward Szłyk.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ścigała, A., Szczęsny, R., Kamedulski, P. et al. Copper nitride/silver nanostructures synthesized via wet chemical reduction method for the oxygen reduction reaction. J Nanopart Res 25, 28 (2023). https://doi.org/10.1007/s11051-023-05671-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05671-z