Abstract
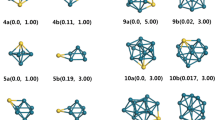
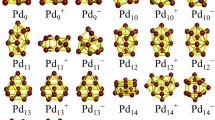
Metallic clusters have been widely studied due to their special electrical, optical, and catalytic properties. The many-body Gupta potential is applied to describe the interatomic interaction of Ni, Cu, Ag, Au, Pd, and Pt clusters, and their global minimal structures within 100 atoms are optimized using dynamic lattice searching (DLS) method. The configurational distribution of global minima is analyzed, and the geometrical difference among these clusters is demonstrated. Results show that the dominant motif of Ni and Cu clusters is the icosahedron, and in Ag and Au clusters the number of decahedra is slightly larger than that of the icosahedra. However, more face-centered cubic (fcc), stacking fault fcc, and amorphous structures are formed in Au clusters than in Ag clusters. Furthermore, the main motif of Pd and Pt clusters is the decahedron. In particular, Ni98 adopts a Leary tetrahedral motif, and Pt54 is a central vacant icosahedron. The difference related to the potential parameters of these metallic clusters is further investigated by energy analysis. Moreover, the potential energy surfaces (PES) of 38-atom metallic clusters is characterized in terms of conformational analysis. It was found that the sequence of the number of local minima on the PES from large to low is Ni, Cu, Ag, Pt, Pd, and Au.






Similar content being viewed by others
References
Aprà E, Baletto F, Ferrando R, Fortunelli A (2004) Amorphization mechanism of icosahedral metal nanoclusters. Phys Rev Lett 93:065502
Baletto F, Ferrando R (2005) Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371–423
Bulusu S, Zeng XC (2006) Structures and relative stability of neutral gold clusters: Au n (n=15–19). J Chem Phys 125:154303
Castro T, Reifenberger R, Choi E, Andres RP (1990) Size-dependent melting temperature of individual nanometer-sized metallic clusters. Phys Rev B 42:8548–8556
Cheng LJ, Cai WS, Shao XG (2004) A connectivity table for cluster similarity checking in the evolutionary optimization method. Chem Phys Lett 389:309–314
Cheng LJ, Yang JL (2007) Modified Morse potential for unification of the pair interactions. J Chem Phys 127:124104
Cleri F, Rosato V (1993) Tight-binding potentials for transition metals and alloys. Phys Rev B 48:22–33
Cleveland CL, Landman U, Schaaff TG, Shafigullin MN, Stephens PW, Whetten RL (1997) Structural evolution of smaller gold nanocrystals: the truncated decahedral motif. Phys Rev Lett 79:1873–1876
Deaven DM, Tit N, Morris JR, Ho KM (1996) Structural optimization of Lennard-Jones clusters by a genetic algorithm. Chem Phys Lett 256:195–200
Doye JPK (2006) Lead clusters: different potentials, different structures. Comput Mater Sci 35:227–231
Doye JPK, Miller MA, Wales DJ (1999) Evolution of the potential energy surface with size for Lennard-Jones clusters. J Chem Phys 111:8417–8428
Doye JPK, Wales DJ (1998) Global minima for transition metal clusters described by Sutton–Chen potentials. New J Chem 22:733–744
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910
Garzón IL, Michaelian K, Beltrán MR, Posada-Amarillas A, Ordejón P, Artacho E, Sánchez-Portal D, Soler JM (1998) Lowest energy structures of gold nanoclusters. Phys Rev Lett 81:1600–1603
Grigoryan VG, Alamanova D, Springborg M (2005) Structure and energetics of nickel, copper, and gold clusters. Eur Phys J D 34:187–190
Grigoryan VG, Alamanova D, Springborg M (2006) Structure and energetics of Cu N clusters with (2 ≤ N ≤ 150): an embedded-atom-method study. Phys Rev B 73:115415
Husic BE, Schebarchov D, Wales DJ (2016) Impurity effects on solid–solid transitions in atomic clusters. Nano 8:18326–18340
Imaoka T, Kitazawa H, Chun WJ, Omura S, Albrecht K, Yamamoto K (2013) Magic number Pt13 and misshapen Pt12 clusters: which one is the better catalyst? J Am Chem Soc 135:13089–13095
Jiang HY, Cai WS, Shao XG (2002) A random tunneling algorithm for the structural optimization problem. Phys Chem Chem Phys 4:4782–4788
Johnston RL (2003) Evolving better nanoparticles: genetic algorithms for optimising cluster geometries. Dalton Trans 22:4193–4207
Laasonen K, Panizon E, Bochicchio D, Ferrando R (2013) Competition between icosahedral motifs in AgCu, AgNi, and AgCo nanoalloys: a combined atomistic–DFT study. J Phys Chem C 117:26405–26413
Leary RH, Doye JPK (1999) Tetrahedral global minimum for the 98-atom Lennard-Jones cluster. Phys Rev E 60:6320
Li XJ, Fu J, Qin Y, Hao SZ, Zhao JJ (2016) Gupta potentials for five HCP rare earth metals. Comput Mater Sci 112:75–79
Liu DC, Nocedal J (1989) On the limited memory BFGS method for large scale optimization. Math Program 45:503–528
Michaelian K, Rendón N, Garzón IL (1999) Structure and energetics of Ni, Ag, and Au nanoclusters. Phys Rev B 60:2000–2010
Miyajima K, Himeno H, Yamada A, Yamamoto H, Mafuné F (2011) Nanoalloy formation of Ta-containing trimetallic small clusters. J Phys Chem A 115:1516–1520
Northby JA (1987) Structure and binding of Lennard-Jones clusters: 13 ≤ N ≤ 147. J Chem Phys 87:6166–6177
Ouyang RH, Xie Y, Jiang DE (2015) Global minimization of gold clusters by combining neural network potentials and the basin-hopping method. Nano 7:14817–14821
Parsina I, Baletto F (2010) Tailoring the structural motif of AgCo nanoalloys: core/shell versus Janus-like. J Phys Chem C 114:1504–1511
Paz-Borbón LO, Mortimer-Jones TV, Johnston RL, Posada-Amarillas A, Barcaro G, Fortunelli A (2007a) Structures and energetics of 98 atom Pd–Pt nanoalloys: potential stability of the Leary tetrahedron for bimetallic nanoparticles. Phys Chem Chem Phys 9:5202–5208
Paz-Borbón LO, Johnston RL, Barcaro G, Fortunelli A (2007b) A mixed structural motif in 34-atom Pd−Pt clusters. J Phys Chem C 111:2936–2941
Piotrowski MJ, Ungureanu CG, Tereshchuk P, Batista KEA, Chaves AS, Guedes-Sobrinho D, Da Silva JLF (2016) Theoretical study of the structural, energetic, and electronic properties of 55-atom metal nanoclusters: a DFT investigation within van der Waals corrections, spin-orbit coupling, and PBE+U of 42 metal systems. J Phys Chem C 120:28844–28856
Qin LJ, Zhang YH, Huang SP, Tian HP, Wang P (2010) Atomic-scale structure of Co-Pt bimetallic nanoparticles: Monte Carlo simulations. Phys Rev B 82:075413
Rapallo A, Olmos-Asar JA, Oviedo OA, Ludueña M, Ferrando R, Mariscal MM (2012) Thermal properties of Co/Au nanoalloys and comparison of different computer simulation techniques. J Phys Chem C 116:17210–17218
Rossi G, Schiappelli G, Ferrando R (2009) Formation pathways and energetic stability of icosahedral AgshellCocore nanoclusters. J Comput Theor Nanosci 6:841–848
Shao XG, Cheng LJ, Cai WS (2004) Dynamic lattice searching method for fast optimization of Lennard-Jones clusters. J Comput Chem 25:1693–1698
Shao XG, Wu X, Cai WS (2009) Dynamic lattice searching methods for optimization of clusters. Front Chem Chin 4:335–342
Shao XG, Wu X, Cai WS (2010) Configuration of the surface atoms in Al N (270 ≤ N ≤ 500) clusters. J Phys Chem A 114:12813–12818
Scott RWJ, Wilson OM, Oh SK, Kenik EA, Crooks RM (2004) Bimetallic palladium-gold dendrimer-encapsulated catalysts. J Am Chem Soc 126:15583–15591
Sebetci A, Güvenç ZB (2005) Global minima of Al N , Au N and Pt N , N ≤ 80, clusters described by the Voter-Chen version of embedded-atom potentials. Modelling Simul Mater Sci Eng 13:683–698
Song W, Lu WC, Wang CZ, Ho KM (2011) Magnetic and electronic properties of the nickel clusters Ni n (n ≤ 30). Comput Theor Chem 978:41–46
Sun YG, Xia YN (2002) Shape-controlled synthesis of gold and silver nanoparticles. Science 298:2176–2179
Takeuchi H (2006) Clever and efficient method for searching optimal geometries of Lennard-Jones clusters. J Chem Inf Model 46:2066–2070
Viñes F, Gomes JRB, Illas F (2014) Understanding the reactivity of metallic nanoparticles: beyond the extended surface model for catalysis. Chem Soc Rev 43:4922–4939
Wales DJ, Doye JPK (1997) Global optimization by basin-hopping and the lowest energy structures of Lennard-Jones clusters containing up to 110 atoms. J Phys Chem A 101:5111–5116
Wille LT (1987) Minimum-energy configurations of atomic clusters: new results obtained by simulated annealing. Chem Phys Lett 133:405–410
Wu X, Dong YJ (2014) Theoretical studies of structures and energies of Pd, Au–Pd, and Au-Pd-Pt clusters. New J Chem 38:4893–4900
Wu X, Dong YJ (2015) Geometrical structures and energetics of gold clusters from Au13 to Au300. Struct Chem 26:393–400
Wu X, Sun Y, Wei Z, Chen TJ (2017) Influence of noble metal dopants (M= Ag, Au, Pd or Pt) on the stable structures of bimetallic Co-M clusters. J Alloys Compd 701:447–455
Wu X, Wei Z, Sun Y, Feng YL, Liu QM (2016) Influence of the potential model parameters on the structures and potential energy surface of cobalt clusters. Chem Phys Lett 660:11–17
Yang XL, Cai WS, Shao XG (2007a) A dynamic lattice searching method with constructed core for optimization of large Lennard-Jones clusters. J Comput Chem 28:1427–1433
Yang XL, Cai WS, Shao XG (2007b) Structural variation of silver clusters from Ag13 to Ag160. J Phys Chem A 111:5048–5056
Zhao Z, Fisher A, Cheng DJ (2016) Phase diagram and segregation of Ag–Co nanoalloys: insights from theory and simulation. Nanotechnology 27:115702
Acknowledgements
This study is supported by the National Natural Science Foundation of China (NNSFC) (Grant No. 21203002) and the Key University Science Research Project of Anhui Province (Grand No. KJ2017A349).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, X., Sun, Y. Stable structures and potential energy surface of the metallic clusters: Ni, Cu, Ag, Au, Pd, and Pt. J Nanopart Res 19, 201 (2017). https://doi.org/10.1007/s11051-017-3907-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3907-6