Abstract
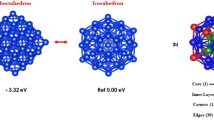
Bimetallic core–shell nanoparticles (CSNPs) have attracted great interest not only because of their superior stability, selectivity, and catalytic activity but also due to their tunable properties achieved by changing the morphology, sequence, and sizes of both core and shell. In this study, the structure, stability, charge transfer, electronic, and magnetic properties of 13-atom and 55-atom Cu and Cu–Ni CSNPs were investigated using the density functional theory (DFT) calculations. The results show that Ni@Cu CSNPs with a Cu surface shell are more energetically favorable than Cu@Ni CSNPs with a Ni surface shell. Interestingly, three-shell Ni@Cu12@Ni42 is more stable than two-shell Cu13@Ni42, while two-shell Ni13@Cu42 is more stable than three-shell Cu@Ni12@Cu42. Analysis of Bader charge illustrates that the charge transfer increases from Cu core to Ni shell in Cu@Ni NPs, while it decreases from Ni core to Cu shell in Ni@Cu NPs. Furthermore, the charge transfer results that d-band states have larger shift toward the Fermi level for the Ni@Cu CSNPs with Cu surface shell, while the Cu@Ni CSNPs with Ni surface shell have similar d-band state curves and d-band centers with the monometallic Ni NPs. In addition, the Cu–Ni CSNPs possess higher magnetic moment when the Ni atoms aggregated at core region of CSNPs, while having lower magnetic moment when the Ni atoms segregate on surface region. The change of the Cu atom location in CSNPs has a weak effect on the total magnetic moment. Our findings provide useful insights for the design of bimetallic core–shell catalysts.






Similar content being viewed by others
References
Austin N, Butina B, Mpourmpakis G (2016) CO2 activation on bimetallic CuNi nanoparticles. Progress in Natural Science: Materials International 26:487–492. doi:10.1016/j.pnsc.2016.08.007
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Borjesson A, Zhu WM, Amara H, Bichara C, Bolton K (2009) Computational studies of metal-carbon nanotube interfaces for regrowth and electronic transport. Nano Lett 9:1117–1120. doi:10.1021/nl8036245
Boyukata M, Belchior JC (2008) Structural and energetic analysis of copper clusters: MD study of Cu-n (n = 2-45). J Braz Chem Soc 19:884–893
Carroll KJ, Calvin S, Ekiert TF, Unruh KM, Carpenter EE (2010) Selective nucleation and growth of Cu and Ni core/shell nanoparticles. Chem Mater 22:2175–2177. doi:10.1021/cm1004032
Chatterjee J, Bettge M, Haik Y, Chen CJ (2005) Synthesis and characterization of polymer encapsulated Cu-Ni magnetic nanoparticles for hyperthermia applications. J Magn Magn Mater 293:303–309. doi:10.1016/j.jmmm.2005.02.024
Chen WP, Li LT, Qi JQ, Wang Y, Gui ZL (1998) Influence of electroless nickel plating on multilayer ceramic capacitors and the implications for reliability in multilayer ceramic capacitors. J Am Ceram Soc 81:2751–2752
Damianos K, Solokha P, Ferrando R (2013) Core-shell and matryoshka structures in MgNi nanoalloys: a computational study. RSC Adv 3:9419–9430. doi:10.1039/c3ra40861b
Ding F, Larsson P, Larsson JA, Ahuja R, Duan HM, Rosen A, Bolton K (2008) The importance of strong carbon-metal adhesion for catalytic nucleation of single-walled carbon nanotubes. Nano Lett 8:463–468. doi:10.1021/nl072431m
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910. doi:10.1021/cr040090g
Gawande MB et al (2015) Core-shell nanoparticles: synthesis and applications in catalysis and electrocatalysis. Chem Soc Rev 44:7540–7590. doi:10.1039/c5cs00343a
Guisbiers G, Khanal S, Ruiz-Zepeda F, de la Puente JR, Jose-Yacaman M (2014) Cu-Ni nano-alloy: mixed, core-shell or Janus nano-particle? Nanoscale 6:14630–14635. doi:10.1039/c4nr05739b
Guo HZ, Jin JR, Chen YZ, Liu X, Zeng DQ, Wang LS, Peng DL (2016) Controllable synthesis of Cu-Ni core-shell nanoparticles and nanowires with tunable magnetic properties. Chem Commun 52:6918–6921. doi:10.1039/c6cc02868c
Guo L, Li AX, An XY, Cao ZR, Liu NY (2015) Catalytic activity of TM@Cu-12 core shell nanoclusters for water gas shift reaction. Int J Hydrog Energy 40:8330–8340. doi:10.1016/j.ijhydene.2015.04.120
Henkelman G, Arnaldsson A, Jónsson H (2006) A fast and robust algorithm for Bader decomposition of charge density. Comput Mater Sci 36:354–360. doi:10.1016/j.commatsci.2005.04.010
Hibino T, Hashimoto A, Inoue T, Tokuno J, Yoshida S, Sano M (2000) A low-operating-temperature solid oxide fuel cell in hydrocarbon-air mixtures. Science 288:2031–2033. doi:10.1126/science.288.5473.2031
Knickelbein MB (2001) Electric dipole polarizabilities of Ni12-58. J Chem Phys 115:5957–5964
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50. doi:10.1016/0927-0256(96)00008-0
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775
Kuznetsov AA et al (2007) Local radiofrequency-induced hyperthermia using CuNi nanoparticles with therapeutically suitable Curie temperature. J Magn Magn Mater 311:197–203. doi:10.1016/j.jmmm.2006.11.199
Li ZW, Li M, Bian ZF, Kathiraser Y, Kawi S (2016) Design of highly stable and selective core/yolk-shell nanocatalysts—a review. Applied Catalysis B-Environmental 188:324–341. doi:10.1016/j.apcatb.2016.01.067
Lin JH, Guliants VV (2012) Alumina-supported Cu@Ni and Ni@Cu core-shell nanoparticles: synthesis, characterization, and catalytic activity in water-gas-shift reaction. Applied Catalysis a-General 445:187–194. doi:10.1016/j.apcata.2012.08.013
Lin J-H, Biswas P, Guliants VV, Misture S (2010) Hydrogen production by water-gas shift reaction over bimetallic Cu-Ni catalysts supported on La-doped mesoporous ceria. Applied Catalysis a-General 387:87–94. doi:10.1016/j.apcata.2010.08.003
Nikje MMA, Vakili M (2015) Engineered magnetic core-shell structures. Curr Pharm Des 21:5312–5323. doi:10.2174/1381612821666150917093352
Noh SH, Seo MH, Seo JK, Fischer P, Han B (2013) First principles computational study on the electrochemical stability of Pt-Co nanocatalysts. Nanoscale 5:8625–8633. doi:10.1039/c3nr02611f
Ozcelik S, Guvenc ZB (2003) Structures and melting of Cu-n (n = 13, 14, 19, 55, 56) clusters. Surf Sci 532:312–316. doi:10.1016/s0039-6028(03)00432-1
Panizon E, Olmos-Asar JA, Peressi M, Ferrando R (2015) Study of structures and thermodynamics of CuNi nanoalloys using a new DFT-fitted atomistic potential. Phys Chem Chem Phys 17:28068–28075. doi:10.1039/c5cp00215j
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Piotrowski MJ, Piquini P, Da Silva JLF (2012) Platinum-based nanoalloys PtnTM55-n (TM = Co, Rh, Au): a density functional theory investigation. J Phys Chem C 116:18432–18439. doi:10.1021/jp302844f
Purbia R, Paria S (2015) Yolk/shell nanoparticles: classifications, synthesis, properties, and applications. Nanoscale 7:19789–19873. doi:10.1039/c5nr04729c
Rapallo A, Olmos-Asar JA, Oviedo OA, Luduena M, Ferrando R, Mariscal MM (2012) Thermal properties of Co/Au nanoalloys and comparison of different computer simulation techniques. J Phys Chem C 116:17210–17218. doi:10.1021/jp302001c1
Reshetenko TV, Avdeeva LB, Ismagilov ZR, Chuvilin AL, Ushakov VA (2003) Carbon capacious Ni-Cu-Al2O3 catalysts for high-temperature methane decomposition. Applied Catalysis a-General 247:51–63. doi:10.1016/s0926-860x(03)00080-2
Sahoo S, Rollmann G, Entel P (2006) Segregation and ordering in binary transition metal clusters. Phase Transit 79:693–700. doi:10.1080/01411590600961164
Sanville E, Kenny SD, Smith R, Henkelman G (2007) Improved grid-based algorithm for Bader charge allocation. J Comput Chem 28:899–908. doi:10.1002/jcc.20575
Singh R, Kroll P (2008) Structural, electronic, and magnetic properties of 13-, 55-, and 147-atom clusters of Fe, Co, and Ni: a spin-polarized density functional study. Phys Rev B 78. doi:10.1103/PhysRevB.78.245404
Songping W, Li J, Jing N, Zhenou Z, Song L (2007) Preparation of ultra fine copper-nickel bimetallic powders for conductive thick film. Intermetallics 15:1316–1321. doi:10.1016/j.intermet.2007.04.001
Sun Q, Zhang XQ, Wang Y, Lu AH (2015) Recent progress on core-shell nanocatalysts. Chin J Catal 36:683–691. doi:10.1016/s1872-2067(14)60298-9
Sun XL, Li DG, Guo SJ, Zhu WL, Sun SH (2016) Controlling core/shell Au/FePt nanoparticle electrocatalysis via changing the core size and shell thickness. Nanoscale 8:2626–2631. doi:10.1039/c5nr06492a
Vizcaino AJ, Carrero A, Calles JA (2007) Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts. Int J Hydrog Energy 32:1450–1461. doi:10.1016/j.ijhydene.2006.10.024
Wang QA, Lim KH, Yang SW, Yang YH, Chen YA (2011) Atomic carbon adsorption on Ni nanoclusters: a DFT study. Theor Chem Accounts 128:17–24. doi:10.1007/s00214-010-0736-4
Wang Q, Wang H, Wei L, Yang S-W, Chen Y (2012) Reactive sites for chiral selective growth of single-walled carbon nanotubes: a DFT study of Ni-55-C-n complexes. J Phys Chem A 116:11709–11717. doi:10.1021/jp308115f
Wei SY, Wang Q, Zhu JH, Sun LY, Lin HF, Guo ZH (2011) Multifunctional composite core-shell nanoparticles. Nanoscale 3:4474–4502. doi:10.1039/c1nr11000d
Xiang Y, Sun DY, Gong XG (2000) Generalized simulated annealing studies on structures and properties of Ni-n (n = 2-55) clusters. J Phys Chem A 104:2746–2751
Xiao BB, Zhu YF, Lang XY, Wen Z, Jiang Q (2014) Al-13@Pt-42 core-shell cluster for oxygen reduction reaction. Scientific Reports 4. doi:10.1038/srep05205
Yamauchi T, Tsukahara Y, Sakata T, Mori H, Yanagida T, Kawai T, Wada Y (2010) Magnetic Cu-Ni (core-shell) nanoparticles in a one-pot reaction under microwave irradiation. Nanoscale 2:515–523. doi:10.1039/b9nr00302a
Yang ZX, Zhang YX, Wu RQ (2012) High stability and reactivity of Pt-based core-shell nanoparticles for oxygen reduction reaction. J Phys Chem C 116:13774–13780. doi:10.1021/jp300971e
Yang ZM, Wang Q, Shan XY, Li WQ, Chen GH, Zhu HJ (2015) DFT study of Fe-Ni core-shell nanoparticles: stability, catalytic activity, and interaction with carbon atom for single-walled carbon nanotube growth. J Chem Phys 142. doi:10.1063/1.4907897
Zhang MH, Wang N, Yang YP, Huang SP (2015) Insight into the relationship between structure and magnetic properties in icosahedral FenPt55-n (n = 0-55) nanoparticles: DFT approach. Journal of Molecular Graphics & Modelling 62:294–302. doi:10.1016/j.jmgm.2015.10.010
Zhao B, Shao G, Fan BB, Zhao WY, Zhang R (2015a) Preparation and electromagnetic wave absorption properties of novel dendrite-like NiCu alloy composite. RSC Adv 5:42587–42590. doi:10.1039/c5ra05323d
Zhao B, Zhao WY, Shao G, Fan BB, Zhang R (2015b) Morphology-control synthesis of a core-shell structured NiCu alloy with tunable electromagnetic-wave absorption capabilities Acs. Appl Mater Interfaces 7:12951–12960. doi:10.1021/acsami.5b02716
Acknowledgments
This work was supported by the National Natural Science Foundations of China (21203094, 21373112) and China Postdoctoral Science Foundation (2016M591834).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Natural Science Foundations of China (21203094, 21373112) and China Postdoctoral Science Foundation (2016M591834).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 28 kb.)
Rights and permissions
About this article
Cite this article
Wang, Q., Wang, X., Liu, J. et al. Cu–Ni core–shell nanoparticles: structure, stability, electronic, and magnetic properties: a spin-polarized density functional study. J Nanopart Res 19, 25 (2017). https://doi.org/10.1007/s11051-016-3731-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3731-4