Abstract
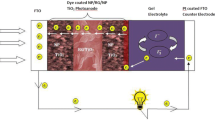
A new facile strategy for fabrication of high surface area electrode in the form of mixtures of coated carbon nanotubes (CNTs) and TiO2 nanoparticles with various weight ratios is reported. The so-called polymeric gel process was used to deposit thick film containing uniform distribution of TiO2 nanoparticles and coated CNTs with high porosity by dip coating for dye-sensitized solar cells (DSSCs) applications. Based on simultaneous differential thermal analysis, the minimum annealing temperature to obtain inorganic- and organic-free films was determined at 500 °C. X-ray diffraction analysis revealed that deposited films were composed of primary nanoparticles with crystallite size in the range 21–45 nm. Field emission scanning electron microscope images showed that deposited films had porous morphology containing uniform spherical particles with diameter around 2.5 μm and coated CNTs with TiO2 nanoparticles (TNTs). The spherical particles were composed of small nanoparticles (~60 nm), improving light scattering and dye loading of the DSSC. Moreover, TNTs were uniformly incorporated into the electrodes, improving electron transportation. X-ray photoelectron spectroscopy was presented as an obvious proof for the inclusion of CNTs into the TiO2 matrix. Ultraviolet–visible spectroscopy showed that CNT introduction enhanced the visible light absorption of photoanode by shifting the absorption onset to visible light region. An enhancement of power conversion efficiency (PCE) from 6.53 % for pure TiO2 to 7.38 % for CNT–TiO2 electrode containing 0.025 wt% CNTs was achieved. This well-incorporated study would present an intellectual development in the fabrication of low-cost CNT consolidated DSSCs with high PCE.









Similar content being viewed by others
References
Ago H, Kugler T, Cacialli F et al (1999) Work functions and surface functional groups of multiwall carbon nanotubes. J Phys Chem B 103:8116–8121
Bakhshayesh AM, Mohammadi MR (2012) Development of nanostructured porous TiO2 thick film with uniform spherical particles by a new polymeric gel process for dye-sensitized solar cell applications. Electrochim Acta 89:90–97
Bond AM, Miao W, Raston CL (2000) Mercury(II) immobilized on carbon nanotubes: synthesis, characterization, and redox properties. Langmuir 16:6004–6012
Chen LC, Ho YC, Guo WS et al (2009) Enhanced visible light-induced photoelectrocatalytic degradation of phenol by carbon nanotube-doped TiO2 electrodes. Electrochim Acta 54:3884–3891
Cullity BD, Stock SR (2001) Elements of X-ray diffraction. Prentice Hall, Lawrence
Eder D, Windle AH (2008) Carbon–inorganic hybrid materials: the carbon-nanotube/TiO2 interface. Adv Mater 20:1787–1793
Feigenbrugel V, Loew C, Calvé SL, Mirabel P (2005) Near-UV molar absorptivities of acetone, alachlor, metolachlor, diazinon and dichlorvos in aqueous solution. J Photochem Photobiol A 174:76–81
Guimin A, Wanhong M, Zhenyu S et al (2007) Preparation of titania/carbon nanotube composites using supercritical ethanol and their photocatalytic activity for phenol degradation under visible light irradiation. Carbon 45:1795–1801
Hagfeldt A, Grätzel M (2000) Synthesis of TiO2 nanoparticles on plasma-treated carbon nanotubes and its application in photoanodes of dye-sensitized solar cells molecular photovoltaics. Acc Chem Res 33:269–277
Ito S, Liska P, Pechy P et al (2005) Control of dark current in photoelectrochemical (TiO2/I−–I3−) and dye-sensitized solar cells. Chem Commun 34:4351–5453
Ito S, Murakami N, Comte P et al (2008) Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10. Thin Solid Films 516:4613–4619
Lin WJ, Hsu CT, Tsai UC (2011) Dye-sensitized solar cells based on multiwalled carbon nanotube-titania/titania bilayer structure photoelectrode. J Colloid Interface Sci 358:562–566
Morales ER, Mathews NR, Reyes-Coronado D et al (2012) Physical properties of the CNT:TiO2 thin films prepared by sol–gel dip coating. Sol Energy 86:1037–1044
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Sawatsuk T, Chindaduang A, Sae-kung C (2009) Dye-sensitized solar cells based on TiO2–MWCNTs composite electrodes: performance improvement and their mechanisms. Diam Relat Mater 18:524–527
Song Z, Hrbek J, Osgood R (2005) Formation of TiO2 nanoparticles by reactive-layer-assisted deposition and characterization by XPS and STM. Nano Lett 5(7):1327–1332
Spurr R, Myers H (1957) Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal Chem 29:760–762
Sun S, Gao L, Liu Y (2011) Optimization of the cutting process of multi-wall carbon nanotubes for enhanced dye-sensitized solar cells. Thin Solid Films 519:2273–2279
Tan B, Wu Y (2006) Dye-sensitized solar cells based on anatase TiO2 nanoparticle/nanowire composites. J Phys Chem B 110:15932–15938
Tsai TH, Chiou SC, Chen SM (2011) Enhancement of dye-sensitized solar cells by using Graphene–TiO2 composites as photoelectrochemical working electrode. Int J Electrochem Sci 63:3333–3343
Wang W, Serp P, Kalck P, Faria JL (2005) Visible light photodegradation of phenol on MWNT–TiO2 composite catalysts prepared by a modified sol–gel method. J Mol Catal A 235:194–199
Xie Y, Heo SH, Yoo SH et al (2010) Synthesis and photocatalytic activity of anatase TiO2 nanoparticles-coated carbon nanotubes. Nanoscale Res Lett 5:603–607
Yella A, Lee HW, Tsao HN et al (2011) Prophyrin-sensitized solar cells with cobalt (II–III)-based redox electrolyte exceed 12 percent efficiency. Science 334:629–634
Zhang W, Cui XL, Jiang ZY (2008) Effect of composite modes on photoelectrochemical properties of MWCNTs/TiO2 nanocomposite films. Acta Phys Chim Sin 24:1975–1980
Zhang S, Niu H, Cheng C, Lan Y et al (2011) Synthesis of TiO2 nanoparticles on plasma-treated carbon nanotubes and its application in photoanodes of dye-sensitized solar Cells. J Phys Chem C 115:22025–22034
Acknowledgments
The authors wish to thank the financial support from Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakhshayesh, A.M., Mohammadi, M.R., Masihi, N. et al. Improved electron transportation of dye-sensitized solar cells using uniform mixed CNTs–TiO2 photoanode prepared by a new polymeric gel process. J Nanopart Res 15, 1961 (2013). https://doi.org/10.1007/s11051-013-1961-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1961-2