Abstract
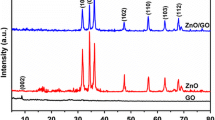
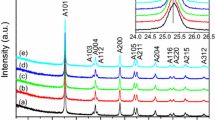
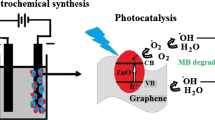
This work reports a simple one-step synthesis of ZnO nanopyramids supported on reduced graphene oxide (RGO) nanosheets using microwave irradiation (MWI) of zinc acetate and GO in the presence of a mixture of oleic acid and oleylamine. The rapid decomposition of zinc acetate by MWI in the presence of the mixture of oleic acid and oleylamine results in the formation of hexagonal ZnO nanopyramids. GO has a high affinity for absorbing MWI, which results in a high local heating effect around the GO nanosheets and facilitates the reduction of GO by the oleylamine. The RGO nanosheets act as heterogeneous surface sites for the nucleation and growth of the ZnO nanopyramids. Using ligand exchange, the ZnO–RGO nanocomposites can be dispersed in an aqueous medium, thus allowing their use as photocatalysts for the degradation of the malachite green dye in water. The ZnO–RGO nanocomposites show enhanced photocatalytic activity for the degradation of the dye over the unsupported ZnO nanopyramids. The enhanced activity is attributed to efficient charge transfer of the photogenerated electrons in the conduction band of ZnO to graphene. This enhances the oxidative pathway of the holes generated in the valence band of ZnO which can effectively lead to the degradation and mineralization of the malachite green. The ZnO nanopyramids supported on RGO could have improved performance in other photocatalytic reactions and also in solar energy conversion.









Similar content being viewed by others
References
Abdelsayed V, Panda AB, Glaspell GP, El-Shall MS (2008) Nanoparticles: synthesis, stabilization, passivation, and functionalization. In: Nagarajan R and Hatton TA (eds) ACS Symposium Series 996, Chap. 17, 225–247
Abdelsayed V, Aljarash A, El-Shall MS, Al Othman ZA, Alghamdi AH (2009) Microwave synthesis of bimetallic nanoalloys and CO oxidation on ceria-supported nanoalloys. Chem Mater 21:2825–2834
Allen MJ, Tung VC, Kaner RB (2009) Honeycomb carbon: a review of graphene. Chem Rev 110:132–145
Banhart F, Kotakoski J, Krashennnikov AV (2011) Structural defects in graphene. ACS Nano 5:26–41
Bilecka I, Elser P, Niederberger M (2009) Kinetic and thermodynamic aspects in the microwave-assisted synthesis of zno nanoparticles in benzyl alcohol. ACS Nano 3:467–477. doi:10.1021/nn800842b
Cancado LG, Jorio A, Martins Ferreira EH, Stavale F, Achete CA, Capaz RB, Moutinho MVO, Lombardo A, Kulmala TS, Ferrari AC (2011) Quantifying defects in graphene via raman spectroscopy at different excitation energies. Nano Lett 11:3190–3196
Chang JF, Kuo HH, Leu IC, Hon MH (2002) The effects of thickness and operation temperature on ZnO: Al thin film CO gas sensor. Sens Actuators B Chem 84:258–264
Chen YL, Hu ZA, Chang YQ, Wang HW, Zhang ZY, Yang YY et al (2011) Zinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrix. J Phys Chem C 115:2563–2571. doi:10.1021/jp109597n
Comini E, Faglia G, Sberveglieri G, Pan ZW, Wang ZL (2002) Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl Phys Lett 81:1869–1871. doi:10.1063/1.1504867
Conner WC, Tompsett GA (2008) How could and do microwaves influence chemistry at interfaces? J Phys Chem B 112:2110–2118. doi:10.1021/jp0775247
Cuong TV, Pham VH, Chung JS, Shin EW, Yoo DH, Hahn SH et al (2010) Solution-processed ZnO-chemically converted graphene gas sensor. Mater Lett 64:2479–2482. doi:10.1016/j.matlet.2010.08.027
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F et al (2009) Raman spectrum of graphene and graphene layers. Phys Rev Lett 9:187401. doi:10.1103/PhysRevLett.97.187401
Geim AK (2009) Graphene: status and prospects. Science 324:1530–1534
Goncalves G, Marques PAAP, Granadeiro CM, Nogueira HIS, Singh MK, Gracio J (2009) Surface modification of graphene nanosheets with gold nanoparticles: the role of oxygen moieties at graphene surface on gold nucleation and growth. Chem Mater 21:4796–4802
Gopalakrishnan K, Joshi HM, Kumar P, Panchakarla LS, Rao CNR (2011) Selectivity in the photocatalytic properties of the composites of TiO2 nanoparticles with B- and N-doped graphenes. Chem Phys Lett 511:304–308. doi:10.1016/j.cplett.2011.06.033
Hassan HMA, Abdelsayed V, Khder AER, AbouZeid KM, Terner J, El-Shall MS, Al-Resayes SI, El-Azhary AA (2009) Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J Mater Chem 19:3832–3837
Herring NP, AbouZeid K, Mohamed MB, Pinsk J, El-Shall MS (2011) Formation mechanisms of gold–zinc oxide hexagonal nanopyramids by heterogeneous nucleation using microwave synthesis. Langmuir 27:15146–15154. doi:10.1021/la201698k
Herring NP, Panda AB, AbouZeid KM, Olson C, Patel A, El-Shall MS (2013) Microwave synthesis of metal oxide nanoparticles. In: Metal oxide nanomaterials for chemical sensors. Series title 7427. Springer, New York
Hu X, Li G, Yu JC (2010) Design, fabrication, and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir 26:3031–3039. doi:10.1021/la902142b
Huang MH, Mao S, Feick H, Yan HQ, Wu YY, Kind H et al (2001) Room-temperature ultraviolet nanowire nanolasers. Science 292:1897–1899
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339. doi:10.1021/ja01539a017
Jagadish C, Pearton SJ (2006) Zinc oxide bulk, thin films and nanostructures. Elsevier, New York
Kamat PV (2010) Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J Phys Chem Lett 1:520–527. doi:10.1021/jz900265j
Kerr LL, Li XN, Canepa M, Sommer AJ (2007) Raman analysis of nitrogen doped ZnO. Thin Solid Films 515:5282–5286. doi:10.1016/j.tsf.2006.12.186
Kim G, Jhi S-H (2011) Carbon monoxide-tolerant platinum nanoparticle catalysts on defect-engineered graphene. ACS Nano 5:805
Kong YC, Yu DP, Zhang B, Fang W, Feng SQ (2001) Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl Phys Lett 78:407–409
Lee JM, Pyun YB, Yi J, Choung JW, Park WI (2009) ZnO nanorod-graphene hybrid architectures for multifunctional conductors. J Phys Chem C 113:19134–19138. doi:10.1021/jp9078713
Lidstrom P, Tierney J, Wathey B, Westman J (2001) Microwave assisted organic synthesis—a review. Tetrahedron 57:9225–9283
Lin Y, Zhang K, Chen W, Liu Y, Geng Z, Zeng J, Pan N, Yan L, Wang W, Hou JG (2010) Dramatically enhanced photoresponse of reduced graphene oxide with linker-free anchored CdSe nanoparticles. ACS Nano 4:3033–3038. doi:10.012/nn100134j
Liu J, Jeong H, Liu J, Lee K, Park J-Y, Ahn YH et al (2010) Reduction of functionalized graphite oxides by trioctylphosphine in non-polar organic solvents. Carbon 48:2282–2289. doi:10.1016/j.carbon.2010.03.002
Lupan O, Emelchenko GA, Ursaki VV, Chai G, Redkin AN, Gruzintsev AN et al (2010) Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater Res Bull. 45:1026–1032. doi:10.1016/j.materresbull.2010.03.027
Lv T, Pan LK, Liu XJ, Lu T, Zhu G, Sun Z (2011) Enhanced photocatalytic degradation of methylene blue by ZnO-reduced graphene oxide composite synthesized via microwave-assisted reaction. J Alloy Compd 509:10086–10091. doi:10.1016/j.jallcom.2011.08.045
Minne SC, Manalis SR, Quate CF (1995) Parallel atomic force microscopy using cantilevers with integrated piezoresistive sensors and integrated piezoelectric actuators. Appl Phys Lett 67:3918–3920
Mohamed MB, AbouZeid KM, Abdelsayed V, Aljarash AA, El-Shall MS (2010) Growth mechanism of anisotropic gold nanocrystals via microwave synthesis: formation of dioleamide by gold nanocatalysis. ACS Nano 4:2766–2772
Moussa S, Abdelsayed V, El-Shall MS (2011) Laser synthesis of Pt, Pd, CoO and Pd-CoO nanoparticle catalysts supported on graphene. Chem Phys Lett 510:179–184
Moussa S, Siamaki AR, Gupton BF, El-Shall MS (2012) Pd-partially reduced graphene oxide catalysts (Pd/PRGO): laser synthesis of Pd nanoparticles supported on PRGO nanosheets for carbon–carbon cross coupling reactions. ACS Catal 2:145–154. doi:10.1021/cs200497e
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson ML, Grigorieva IV, Dubonos SV, Firsov AA (2005) Two-dimensional gas of massless dirac fermions in graphene. Nature 438:197–200
Panda AB, Glaspell G, El-Shall MS (2006) Microwave synthesis of highly aligned ultra narrow semiconductor rods and wires. J Am Chem Soc 128:2790–2791
Panda AB, Glaspell G, El-Shall MS (2007) Microwave synthesis and optical properties of uniform nanorods and nanoplates of rare earth oxides. J Phys Chem C 111:1861–1864
Pearton SJ, Norton DP, Ip K, Heo YW, Steiner T (2005) Recent progresses in processing and properties of ZnO. Prog Mater Sci 50:293–340. doi:10.1016/j.pmatsci.2004.04.001
Rao CNR, Sood AK, Subrahmanyam KS, Govindaraj A (2009) Graphene: the new two dimensional nanomaterial. Angew Chem Int Ed 48:7752–7777
Raula M, Rashid MH, Paira TK, Dinda E, Mandal TK (2010) Ascorbate-assisted growth of hierarchical ZnO nanostructures: sphere, spindle, and flower and their catalytic properties. Langmuir 26:8769–8782. doi:10.1021/la904507q
Scheuermann GM, Rumi L, Steurer P, Bannwarth W, Mülhaupt R (2009) Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki–Miyaura coupling reaction. J Am Chem Soc 131:8262
Siamaki AR, Khder AERS, Abdelsayed V, El-Shall MS, Gupton BF (2011) Microwave-assisted synthesis of palladium nanoparticles supported on graphene: a highly active and recyclable catalyst for carbon–carbon cross-coupling reactions. J Catal 279:1–11
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys Condens Matter 16:R829–R858. doi:10.1088/0953-8984/16/25/R01
Watts JF, Wolstenholme J (2003) An introduction to surface analysis by XPS and AES. Wiley, New York
Williams G, Kamat PV (2009) Graphene-semiconductor nanocomposites: excited-state interactions between ZnO nanoparticles and graphene oxide. Langmuir 25:13869–13873. doi:10.1021/la900905h
Williams G, Seger B, Kamat PV (2008) TiO(2)-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2:1487–1491. doi:10.1021/nn800251f
Wu SX, Yin ZY, He QY, Huang XA, Zhou XZ, Zhang H (2010) Electrochemical deposition of semiconductor oxides on reduced graphene oxide-based flexible, transparent, and conductive electrodes. J Phys Chem C 114:11816–11821. doi:10.1021/jp103696u
Xu TG, Zhang LW, Cheng HY, Zhu YF (2011) Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl Catal B Environ 101:382–387. doi:10.1016/j.apcatb.2010.10.007
Yang D, Velamakanni A, Bozoklu G, Park S, Stoller M, Piner RD et al (2009) Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 47:145–152. doi:10.1016/j.carbon.2008.09.045
Yin ZY, Wu SX, Zhou XZ, Huang X, Zhang QC, Boey F et al (2010) Electrochemical deposition of ZnO Nanorods on transparent reduced graphene oxide electrodes for hybrid solar cells. Small 6:307–312. doi:10.1002/smll.200901968
Zedan AF, Sappal S, Moussa S, El-Shall MS (2010) Ligand-controlled microwave synthesis of cubic and hexagonal CdSe nanocrystals supported on graphene. Photoluminescence quenching by graphene. J Phys Chem C 114:19920–19927
Zhang JW, Zhu PL, Li JH, Chen JM, Wu ZS, Zhang ZJ (2009) Fabrication of octahedral-shaped polyol-based zinc alkoxide particles and their conversion to octahedral polycrystalline ZnO or single-crystal ZnO nanoparticles. Cryst Growth Des 9:2329–2334. doi:10.1021/cg8012156
Zheng MJ, Zhang LD, Li GH, Shen WZ (2002) Fabrication and optical properties of large-scale uniform zinc oxide nanowire arrays by one-step electrochemical deposition technique. Chem Phys Lett 363:123–128
Acknowledgments
We thank the National Science Foundation (CHE-0911146, OISE-1002970 and CHE-0922582) for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the topical collection on nanomaterials in energy, health and environment
Rights and permissions
About this article
Cite this article
Herring, N.P., Almahoudi, S.H., Olson, C.R. et al. Enhanced photocatalytic activity of ZnO–graphene nanocomposites prepared by microwave synthesis. J Nanopart Res 14, 1277 (2012). https://doi.org/10.1007/s11051-012-1277-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1277-7