Abstract
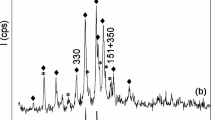
Synthetic sodium type A zeolite bearing Fe2O3 and Fe3O4 nanoparticles composites have been prepared by means of a coprecipitation method with two different activation methodologies, one using Sn and the other using Sn/Pd nanoparticles as activators. Sn activation generates hematite nanoparticles while Sn/Pd produces magnetite nanoparticles. Amount of HCl used during the activation of the zeolite with SnCl2 showed a correlation between the stannous activating species and the particle size. Both Sn and Sn–Pd activated nanocomposites show nearly narrow size distributions but only those iron oxides obtained with Sn–Pd showed supermagnetism.








Similar content being viewed by others
References
Abe H, Govindachetty S, Xu Y, Sekido N, Yamabe-Mitarai Y, Shimoda M (2010) Synthesis and catalytic performance of intermetallic nanoparticles. Materia 49:314–316 ISSN: 1340-2625
Chen D, Chen P, Huagong XC (2009) Synthesis and application of magnetic magnetite nanoparticles. N Chemical Mater 37(49):10–12 ISSN: 1006-3536
Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek (2010) Magnetic nanoparticles and targeted drug delivering. J Pharmacol Res 62:144–149. doi:10.1016/j.phrs.2010.01.014
Christmann K, Schwede S, Schubert S, Kudernatsch W (2010) Model studies on CO oxidation catalyst systems: titania and gold nanoparticles. Chem Phys Chem 11:1344–1363. doi:10.1002/cphc.200900769
Colston SL, O’Connor D, Barnes P, Mayes EL, Mann S, Ereimuth H, Ehrfeld W (2000) Functional micro-concrete: the incorporation of zeolites and inorganic nano-particles into cement micro-structures. J Mater Sci Lett 19:1085–1088. doi:10.1023/A:1006767809807
Dlugopolska K, Ruman T, Pogocki D, Danilczuk M (2009) Wiad Chem 63(11-12):1073–1088 ISSN: 0043-5104
Giouroudi I, Kosel J (2010) Recent progress in biomedical applications of magnetic nanoparticles. J Recent Pat Nanotechnol 4:111–118. doi:10.2174/187221010791208795
Han W, Liu G, Xu Y, Wang P, Liu Q (2007) Method for preparing nanoscale granular material for removing heavy metals. Patent Numer 2007.10300310
Hao R, Xing R, Xu Z, Hou Y, Gao S, Sun S (2010) Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv Mater 22:2729–2742. doi:10.1002/adma.201000260
Hu J, Chen GH, Lo-Irene MC (2005a) Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res 39:4528–4536. doi:10.1016/j.watres.2005.05.051
Hu J, Lo-Irene MC, Chen GH (2005b) Fast removal and recovery of Cr(VI) using surface-modified jacobsite (MnFe2O4) nanoparticles. Langmuir 21:11173–11179. doi:10.1021/la051076h
Iwasaki T (2010) Facile synthesis of superparamagnetic magnetite nanoparticles using mechanochemical effect. Kagaku to Kogyo Chem Chem Ind 61:521–525. ISSN: 0451-2014
Jeyadevan B (2010) Present status and prospects of magnetite nanoparticles-based hyperthermia. J Ceram Soc Jpn 118:391–401. doi:org/10.2109/jcersj2.118.391
Kobayashi Y, Salgueiriño-Maceira V, Liz-Marzán LM (2001) Deposition of silver nanoparticles on silica spheres by pretreatment steps in electroless plating. Chem Mater 13:1630–1633. doi:10.1021/cm001240g
Krishnan KM (2010) Biomedical nanomagnetics: a spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans Magn 46:2523–2558. doi:10.1109/TMAG.2010.2046907
Maeda K, Domen K, Shokubai (2010) Development of core/shell-structured noble-metal/Cr2O3 nanoparticles as effective promoters for overall water splitting by particulate photocatalysts. Shokubai 52:160–165 ISSN: 0559-8958
Mahmoudi M, Simchi A, Imani M (2010) Recent advances in surface engineering of superparamagnetic iron oxide nanoparticles for biomedical applications. J Iran Chem Soc 7:S1–S27. doi:10.1007/BF03246181
Pestryakov AN, Petranovskii VP, Kryazhov A, Ozhereliev O, Pfander N, Knop-Gericke A (2004) Study of copper nanoparticles formation on supports of different nature by UV–Vis diffuse reflectance spectroscopy. Chem Phys Lett 385:173–176. doi:10.1016/j.cplett.2003.12.077
Petranovskii V, Gurin V, Bogdanchikova N, Hernandez M-A, Avalos M (2002) A selectivity of zeolite matrices in the Cu(II) reduction process. Stud Surf Sci Catal 141:561–568. doi:org/10.1016/S0167-2991(02)80590-0
Shekhawat GS, Arya V (2008) Nanomedicines: emergence a new era in biomedical sciences. NanoTrends 5:9–20 ISSN 0973-418X
Shen YF, Tang J, Nie ZH, Wang YD, Ren Y, Zuo L (2009a) Tailoring size and structural distortion of Fe3O4 nanoparticles for the purification of contaminated water. Bioresour Technol 100:4139–4146. doi:10.1016/j.biortech.2009.04.004
Shen YF, Tang J, Nie ZH, Wang YD, Ren Y, Zuo L (2009b) Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep Purif Technol 68:312–319. doi:org/10.1016/j.seppur.2009.05.020
Stakheev AY, Mashkovskii IS, Baeva GN, Telegina NS (2010) Specific features of the catalytic behavior of supported palladium nanoparticles in heterogeneous catalytic reactions. Russ J Gen Chem 80:618–629. doi:10.1134/S1070363210030424
Wang Y, Weinstock I (2010) Cation mediated self-assembly of inorganic cluster anion building blocks. A Dalton Trans 39(27):6143–6152. doi:10.1039/C0DT00166J
Acknowledgments
The authors are grateful to the Autonomous University of the State of Mexico (UAEM) for the financial support through the research project 3246/2012CHT. We thank the CID-KUO for the facilities to fulfill this work. We thank Dr. José Israel Betancourt Reyes for his help with VSM experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendoza-Bello, S., Morales-Luckie, R.A., Flores-Santos, L. et al. Size-controlled synthesis of Fe2O3 and Fe3O4 nanoparticles onto zeolite by means of a modified activated-coprecipitation method: effect of the HCl concentration during the activation. J Nanopart Res 14, 1242 (2012). https://doi.org/10.1007/s11051-012-1242-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1242-5