Abstract
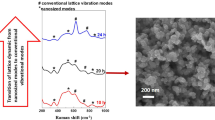
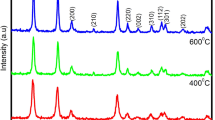
The exploration of novel synthetic methodologies that control both size and shape of functional nanostructure opens new avenues for the functional application of nanomaterials. Here, we report a new and versatile approach to synthesize SnO2 nanocrystals (rutile-type structure) using microwave-assisted hydrothermal method. Broad peaks in the X-ray diffraction spectra indicate the nanosized nature of the samples which were indexed as a pure cassiterite tetragonal phase. Chemically and physically adsorbed water was estimated by TGA data and FT-Raman spectra to account for a new broad peak around 560 cm−1 which is related to defective surface modes. In addition, the spherical-like morphology and low dispersed distribution size around 3–5 nm were investigated by HR-TEM and FE-SEM microscopies. Room temperature PL emission presents two broad bands at 438 and 764 nm, indicating the existence of different recombination centers. When the size of the nanospheres decreases, the relative intensity of 513 nm emission increases and the 393 nm one decreases. UV–Visible spectra show substantial changes in the optical absorbance of crystalline SnO2 nanoparticles while the existence of a small tail points out the presence of localized levels inside the forbidden band gap and supplies the necessary condition for the PL emission.






Similar content being viewed by others
References
Abello L, Bochu B, Gaskov A, Koudryavtseva S, Lucazeau G, Roumyantseva M (1998) Structural characterization of nanocrystalline SnO2 by X-ray and Raman spectroscopy. J Solid State Chem 135(1):78–85
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271(5251):933–937
Baruwati B, Polshettiwar V, Varma RS (2009) Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem 11(7):926–930
Batzill M (2006) Surface science studies of gas sensing materials: SnO2. Sensors 6(10):1345–1366
Bilecka I, Niederberger M (2010) Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2(8):1358–1374
Blattner G, Klingshirn C, Helbig R (1980) Impurity transitions in the photoluminescence spectra of SnO2. Solid State Commun 33(3):341–344
Cai D, Su Y, Chen YQ, Jiang J, He ZY, Chen L (2005) Synthesis and photoluminescence properties of novel SnO2 asterisk-like nanostructures. Mater Lett 59(16):1984–1988. doi:10.1016/j.matlet.2005.01.045
Cao HQ, Qiu XQ, Liang Y, Zhang L, Zhao MJ, Zhu QM (2006) Sol-gel template synthesis and photoluminescence of n- and p-type semiconductor oxide nanowires. ChemPhysChem 7(2):497–501. doi:10.1002/cphc.200500452
Çetin K, Zunger A (2002) Origins of coexistence of conductivity and transparency in SnO2. Phys Rev Lett 88(9):095501
Chang SS, Park DK (2002) Novel Sn powder preparation by spark processing and luminescence properties. Mater Sci Eng B 95(1):55–60
Chen DL, Gao L (2004) Facile synthesis of single-crystal tin oxide nanorods with tunable dimensions via hydrothermal process. Chem Phys Lett 398(1–3):201–206. doi:10.1016/j.cplett.2004.09.055
Chen Y, Zhu J, Zhu X, Ma G, Liu Z, Min N (2003) Gas sensing property and microstructure of SnO2 nanocrystalline prepared by solid state reaction–thermal oxidation. Mater Sci Eng B 99(1–3):52–55
Cheng B, Russell JM, Shi WS, Zhang L, Samulski ET (2004) Large-scale, solution-phase growth of single-crystalline SnO2 nanorods. J Am Chem Soc 126(19):5972–5973
Cullity BD, Stock SR (2001) Elements of X-ray diffraction, 3rd edn. Prentice Hall, Upper Saddle River
Dai ZR, Gole JL, Stout JD, Wang ZL (2002a) Tin oxide nanowires, nanoribbons, and nanotubes. J Phys Chem B 106(6):1274–1279. doi:10.1021/jp013214r
Dai ZR, Pan ZW, Wang ZL (2002b) Growth and structure evolution of novel tin oxide diskettes. J Am Chem Soc 124(29):8673–8680. doi:10.1021/ja026262d
Dal Santos MA, Antunes AC, Ribeiro C, Borges CPF, Antunes SRM, Zara AJ, Pianaro SA (2003) Electric and morphologic properties of SnO2 films prepared by modified sol-gel process. Mater Lett 57(28):4378–4381. doi:10.1016/s0167-577x(03)00328-8
Del Castillo J, Rodriguez VD, Yanes AC, Mendez-Ramos J, Torres ME (2005) Luminescent properties of transparent nanostructured Eu3+ doped SnO2–SiO2 glass–ceramics prepared by the sol–gel method. Nanotechnology 16(5):S300–S303. doi:10.1088/0957-4484/16/5/031
Deng H-X, Li S–S, Li J (2010) Quantum confinement effects and electronic properties of SnO2 quantum wires and dots. J Phys Chem C 114(11):4841–4845. doi:10.1021/jp911035z
Dieguez A, Romano-Rodriguez A, Vila A, Morante JR (2001) The complete Raman spectrum of nanometric SnO2 particles. J Appl Phys 90(3):1550–1557
Dong WS, Li MY, Liu CL, Lin FQ, Liu ZT (2008) Novel ionic liquid assisted synthesis of SnO2 microspheres. J Colloid Interface Sci 319(1):115–122. doi:10.1016/j.jcis.2007.08.031
El-Sayed MA (2004) Small is different: shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Acc Chem Res 37(5):326–333. doi:10.1021/ar020204f
Epifani M, Diaz R, Arbiol J, Comini E, Sergent N, Pagnier T, Siciliano P, Taglia G, Morante JR (2006) Nanocrystalline metal oxides from the injection of metal oxide sols in coordinating solutions: Synthesis, characterization, thermal stabilization, device processing, and gas-sensing properties. Adv Funct Mater 16(11):1488–1498. doi:10.1002/adfm.200500652
Fang M, Tan XL, Cheng BC, Zhang LD (2009) SnO2 hierarchical nanostructure and its strong narrow-band photoluminescence. J Mater Chem 19(9):1320–1324. doi:10.1039/b817530f
Gaidi M, Hajjaji A, Smirani R, Bessais B, El Khakani MA (2010) Structure and photoluminescence of ultrathin films of SnO2 nanoparticles synthesized by means of pulsed laser deposition. J Appl Phys 108(6):063537
Gallis KW, Landry CC (2001) Rapid calcination of nanostructured silicate composites by microwave irradiation. Adv Mater 13(1):23–26. doi:10.1002/1521-4095(200101)13:1<23:aid-adma23>3.0.co;2-9
Gerbec JA, Magana D, Washington A, Strouse GF (2005) Microwave-enhanced reaction rates for nanoparticle synthesis. J Am Chem Soc 127(45):15791–15800. doi:10.1021/ja052463g
Gervais F, Kress W (1985) Lattice-dynamics of oxides with rutile structure and instabilities at the metal-semiconductor phase-Transitions of NbO2 and VO2. Phys Rev B 31(8):4809–4814
Gole JL, Wang ZL (2001) SnOx nanocrystallites supported by silica nanostructures. Nano Lett 1(8):449–451. doi:10.1021/nl010048q
Her YC, Wu JY, Lin YR, Tsai SY (2006) Low-temperature growth and blue luminescence of SnO2 nanoblades. Appl Phys Lett 89(4):3. doi:04311510.1063/1.2235925
Hu JQ, Bando Y, Golberg D (2003a) Self-catalyst growth and optical properties of novel SnO2 fishbone-like nanoribbons. Chem Phys Lett 372(5–6):758–762. doi:10.1016/s0009-2614(03)00503-7
Hu JQ, Bando Y, Liu QL, Golberg D (2003b) Laser-ablation growth and optical properties of wide and long single-crystal SnO2 ribbons. Adv Funct Mater 13(6):493–496. doi:10.1002/adfm.200304327
Huang W, Richert R (2008) The physics of heating by time-dependent fields: microwaves and water revisited. J Phys Chem B 112(32):9909–9913. doi:10.1021/jp8038187
Huang Y, Duan XF, Lieber CM (2005) Nanowires for integrated multicolor nanophotonics. Small 1(1):142–147. doi:10.1002/smll.200400030
Jiang LH, Sun GQ, Zhou ZH, Sun SG, Wang Q, Yan SY, Li HQ, Tian J, Guo JS, Zhou B, Xin Q (2005) Size-controllable synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts. J Phys Chem B 109(18):8774–8778. doi:10.1021/jp050334g
Jouhannaud J, Rossignol J, Stuerga D (2008) Rapid synthesis of tin (IV) oxide nanoparticles by microwave induced thermohydrolysis. J Solid State Chem 181(6):1439–1444. doi:10.1016/j.jssc.2008.02.040
Kappe CO (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43(46):6250–6284. doi:10.1002/anie.200400655
Katiyar RS, Dawson P, Hargreav MM, Wilkinso GRJ (1971) Dynamics of rutile structure 3. Lattice dynamics, infrared and Raman spectra of SnO2. J Phys C 4(15):2421–2431
Kim SW, Fujita S (2002) Self-organized ZnO quantum dots on SiO2/Si substrates by metal organic chemical vapor deposition. Appl Phys Lett 81(26):5036–5038. doi:10.1063/1.1527690
Komarneni S, Roy R, Li QH (1992) Microwave-hydrothermal synthesis of ceramic powders. Mater Res Bull 27(12):1393–1405
Krishna M, Komarneni S (2009) Conventional- vs microwave-hydrothermal synthesis of tin oxide, SnO2 nanoparticles. Ceram Int 35(8):3375–3379. doi:10.1016/j.ceramint.2009.06.010
Kuiri PK, Lenka HP, Ghatak J, Sahu G, Joseph B, Mahapatraa DP (2007) Formation and growth of SnO2 nanoparticles in silica glass by Sn implantation and annealing. J Appl Phys 102(2):5. doi:02431510.1063/1.2761778
Lee EJH, Ribeiro C, Longo E, Leite ER (2006) Growth kinetics of tin oxide nanocrystals in colloidal suspensions under hydrothermal conditions. Chem Phys 328(1–3):229–235. doi:10.1016/j.chemphys.2006.06.032
Leite ER, Weber IT, Longo E, Varela JA (2000) A new method to control particle size and particle size distribution of SnO2 nanoparticles for gas sensor applications. Adv Mater 12(13):965–968
Leite ER, Paris EC, Pontes FM, Paskocimas CA, Longo E, Sensato F, Pinheiro CD, Varela JA, Pizani PS, Campos CEM, Lanciotti F (2003) The origin of photoluminescence in amorphous lead titanate. J Mater Sci 38(6):1175–1178
Li LJ, Zong FJ, Cui XD, Ma HL, Wu XH, Zhang QD, Wang YL, Yang F, Zhao JZ (2007) Structure and field emission properties of SnO2 nanowires. Mater Lett 61(19–20):4152–4155. doi:10.1016/j.matlet.2007.01.044
Liu YK, Zheng CL, Wang WZ, Yin CR, Wang GH (2001a) Synthesis and characterization of rutile SnO2 nanorods. Adv Mater 13(24):1883–1887
Liu YK, Zheng CL, Wang WZ, Zhan YJ, Wang GH (2001b) Production of SnO2 nanorods by redox reaction. J Cryst Growth 233(1–2):8–12
Liu Y, Yang F, Yang X (2008) Size-controlled synthesis and characterization of quantum-size SnO2 nanocrystallites by a solvothermal route. Colloid Surf A 312(2–3):219–225. doi:10.1016/j.colsurfa.2007.06.054
Longo VM, Cavalcante LS, Erlo R, Mastelaro VR, de Figueiredo AT, Sambrano JR, de Lazaro S, Freitas AZ, Gomes L, Vieira ND, Varela JA, Longo E (2008) Strong violet-blue light photoluminescence emission at room temperature in SrZrO3: joint experimental and theoretical study. Acta Mater 56(10):2191–2202. doi:10.1016/j.actamat.2007.12.059
Longo VM, Cavalcante LS, Costa MGS, Moreira ML, de Figueiredo AT, Andres J, Varela JA, Longo E (2009) First principles calculations on the origin of violet–blue and green light photoluminescence emission in SrZrO3 and SrTiO3 perovskites. Theor Chem Acc 124(5–6):385–394. doi:10.1007/s00214-009-0628-7
Luo SH, Chu PK, Liu WL, Zhang M, Lin CL (2006) Origin of low-temperature photoluminescence from SnO2 nanowires fabricated by thermal evaporation and annealed in different ambients. Appl Phys Lett 88(18):3. doi:18311210.1063/1.2201617
Macario LR, Moreira ML, Andres J, Longo E (2010) An efficient microwave-assisted hydrothermal synthesis of BaZrO3 microcrystals: growth mechanism and photoluminescence emissions. CrystEngCommunity 12(11):3612–3619
Majdoub M, Loupy A, Petit A, Roudesli S (1996) Coupling focused microwaves and solvent-free phase transfer catalysis: application to the synthesis of new furanic diethers. Tetrahedron 52(2):617–628
Mao YB, Wong SS (2005) Composition and shape control of crystalline Ca1–xSrxTiO3 perovskite nanoparticles. Adv Mater 17(18):2194–2199. doi:10.1002/adma.200500437
Moreira ML, Pianaro SA, Andrade AVC, Zara AJ (2006) Crystal phase analysis of SnO2-based varistor ceramic using the Rietveld method. Mater Charact 57(3):193–198. doi:10.1016/j.matchar.2006.01.012
Moreira ML, Andres J, Longo VM, Li MS, Varela JA, Longo E (2009) Photoluminescent behavior of SrZrO3/SrTiO3 multilayer thin films. Chem Phys Lett 473(4–6):293–298. doi:10.1016/j.cplett.2009.03.021
Moreira ML, Volanti DP, Andrés J, Montes PJR, Valerio MEG, Varela JA, Longo E (2011) Radioluminescence properties of decaoctahedral BaZrO3. Scripta Mater 64(2):118–121
Orlandi MO, Ramirez AJ, Leite ER, Longo E (2008) Morphological evolution of tin oxide nanobelts after phase transition. Cryst Growth Des 8(3):1067–1072. doi:10.1021/cg7009379
Pan SS, Zhang YX, Teng XM, Li GH, Li L (2008) Optical properties of nitrogen-doped SnO2 films: effect of the electronegativity on refractive index and band gap. J Appl Phys 103(9):093103–093104
Panda AB, Glaspell G, El-Shall MS (2006) Microwave synthesis of highly aligned ultra narrow semiconductor rods and wires. J Am Chem Soc 128(9):2790–2791. doi:10.1021/ja058148b
Paraguay-Delgado F, Antunez-Flores W, Miki-Yoshida M, Aguilar-Elguezaba A, Santiago P, Diaz R, Ascencio JA (2005) Structural analysis and growing mechanisms for long SnO2 nanorods synthesized by spray pyrolysis. Nanotechnology 16(6):688–694. doi:10.1088/0957-4484/16/6/011
Patzke GR, Zhou Y, Kontic R, Conrad F (2010) Oxide nanomaterials: synthetic developments, mechanistic studies, and technological innovations. Angew Chem Int Ed. doi:10.1002/anie.201000235
Pianaro SA, Bueno PR, Longo E, Varela JA (1995) A new SnO2-based varistor system. J Mater Sci Lett 14(10):692–694
Pianaro SA, Bueno PR, Olivi P, Longo E, Varela JA (1998) Electrical properties of the SnO2-based varistor. J Mater Sci Mater Electron 9(2):159–165
Pires FI, Joanni E, Savu R, Zaghete MA, Longo E, Varela JA (2008) Microwave-assisted hydrothermal synthesis of nanocrystalline SnO powders. Mater Lett 62(2):239–242. doi:10.1016/j.matlet.2007.05.006
Pontes FM, Pinheiro CD, Longo E, Leite ER, de Lazaro SR, Magnani R, Pizani PS, Boschi TM, Lanciotti F (2003) Theoretical and experimental study on the photoluminescence in BaTiO3 amorphous thin films prepared by the chemical route. J Lumines 104(3):175–185. doi:10.1016/s0022-2313(03)00014-0
Rabenau A (1985) The role of hydrothermal synthesis in preparative chemistry. Angew Chem Int Ed Engl 24(12):1026–1040
Raghuveer MS, Agrawal S, Bishop N, Ramanath G (2006) Microwave-assisted single-step functionalization and in situ derivatization of carbon nanotubes with gold nanoparticles. Chem Mat 18(6):1390–1393. doi:10.1021/cm051911g
Rao KJ, Vaidhyanathan B, Ganguli M, Ramakrishnan PA (1999) Synthesis of inorganic solids using microwaves. Chem Mat 11(4):882–895
Ribeiro C, Lee EJH, Giraldi TR, Longo E, Varela JA, Leite ER (2004) Study of synthesis variables in the nanocrystal growth behavior of tin oxide processed by controlled hydrolysis. J Phys Chem B 108(40):15612–15617. doi:10.1021/jp0473669
Roduner E (2006) Size matters: why nanomaterials are different. Chem Soc Rev 35(7):583–592. doi:10.1039/b502142c
Scott JF (1970) Raman spectrum of SnO2. J Chem Phys 53(2):852–853
Shek CH, Lin GM, Lai JKL (1999) Effect of oxygen deficiency on the Raman spectra and hyperfine interactions of nanometer SnO2. Nanostruct Mater 11(7):831–835
Strauss CR, Rooney DW (2010) Accounting for clean, fast and high yielding reactions under microwave conditions. Green Chem 12(8):1340–1344
Sun CQ (2010) Dominance of broken bonds and nonbonding electrons at the nanoscale. Nanoscale 2(10):1930–1961. doi:10.1039/c0nr00245c
Sun SH, Meng GW, Wang YW, Gao T, Zhang MG, Tian YT, Peng XS, Zhang LD (2003) Large-scale synthesis of SnO2 nanobelts. Appl Phys A 76(2):287–289. doi:10.1007/s00339-002-1506-5
Trani F, Causà M, Ninno D, Cantele G, Barone V (2008) Density functional study of oxygen vacancies at the SnO2 surface and subsurface sites. Phys Rev B 77(24):245410
Trayler JG, Smith HG, Nicklow RM, Wilkinso MK (1971) Lattice dynamics of rutile. Phys Rev B 3(10):3457–3472
Volanti DP, Keyson D, Cavalcante LS, Simoes AZ, Joya MR, Longo E, Varela JA, Pizani PS, Souza AG (2008) Synthesis and characterization of CuO flower-nanostructure processing by a domestic hydrothermal microwave. J Alloy Compd 459(1–2):537–542. doi:10.1016/j.jallcom.2007.05.023
Volanti DP, Orlandi MO, Andres J, Longo E (2011) Efficient microwave-assisted hydrothermal synthesis of CuO sea urchin-like architectures via a mesoscale self-assembly. CrystEngCommunity 12(6):1696–1699. doi:10.1039/b922978g
Walton RI (2002) Subcritical solvothermal synthesis of condensed inorganic materials. Chem Soc Rev 31:230–238.
Wang ZL (2003) Nanobelts, nanowires, and nanodiskettes of semiconducting oxides—from materials to nanodevices. Adv Mater 15(5):432–436
Wilson GJ, Matijasevich AS, Mitchell DRG, Schulz JC, Will GD (2006) Modification of TiO2 for enhanced surface properties: finite Ostwald ripening by a microwave hydrothermal process. Langmuir 22(5):2016–2027. doi:10.1021/la052716j
Wu DS, Han CY, Wang SY, Wu NL, Rusakova IA (2002) Microwave-assisted solution synthesis of SnO nanocrystallites. Mater Lett 53(3):155–159
Yu KN, Xiong YH, Liu YL, Xiong CS (1997) Microstructural change of nano-SnO2 grain assemblages with the annealing, temperature. Phys Rev B 55(4):2666–2671
Zhao L, Choi M, Kim HS, Hong SH (2007) The effect of multiwalled carbon nanotube doping on the CO gas sensitivity of SnO2-based nanomaterials. Nanotechnology 18(44):5. doi:44550110.1088/0957-4484/18/44/445501
Zhou WZ (2010) Reversed crystal growth: implications for crystal engineering. Adv Mater 22(28):3086–3092. doi:10.1002/adma.200904320
Zhou JX, Zhang MS, Hong JM, Yin Z (2006a) Raman spectroscopic and photoluminescence study of single-crystalline SnO2 nanowires. Solid State Commun 138(5):242–246. doi:10.1016/j.ssc.2006.03.007
Zhou XT, Heigl F, Murphy MW, Sham TK, Regier T, Coulthard I, Blyth RIR (2006b) Time-resolved x-ray excited optical luminescence from SnO2 nanoribbons: direct evidence for the origin of the blue luminescence and the role of surface states. Appl Phys Lett 89(21):3. doi:21310910.1063/1.2387476
Zhu HL, Yang DR, Yu GX, Zhang H, Yao KH (2006) A simple hydrothermal route for synthesizing SnO2 quantum dots. Nanotechnology 17(9):2386–2389. doi:10.1088/0957-4484/17/9/052
Zhu Z, Ouyang G, Yang G (2010) Bandgap shift in SnO2 nanostructures induced by lattice strain and coordination imperfection. J Appl Phys 108(8):083511–083514
Acknowledgments
The authors acknowledge the financial support of the Brazilian research institutions: CAPES, FAPESP, FPTI (Foundation Technological Park of ITAIPU), CNPq, and TEM facilities supplied by LMA-UNESP-Araraquara.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendes, P.G., Moreira, M.L., Tebcherani, S.M. et al. SnO2 nanocrystals synthesized by microwave-assisted hydrothermal method: towards a relationship between structural and optical properties. J Nanopart Res 14, 750 (2012). https://doi.org/10.1007/s11051-012-0750-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0750-7