Abstract
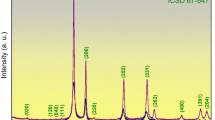
Pulsed laser ablation on Zr plate in water under Q-switch mode and a fluence of 700 and 800 mJ/pulse for a rather high power density of 1.5 and 1.7 × 1011 W/cm2, respectively, was employed to fabricate hydrogenated ZrO2 nanocondensates. X-ray diffraction and transmission electron microscopic observations indicated such nanocondensates are full of {111} and {100} facets and predominantly in monoclinic (m-) rather than cubic- and/or tetragonal (t-) crystal symmetry in particular when fabricated at 700 mJ/pulse. The hydrogenated ZrO2 nanocondensates underwent martensitic t → m transformation at a rather small critical size (ca. 20 nm) due to H+ signature and hence oxygen vacancy deficiency in the lattice. The resultant m-phase was free of twin and fault due to site saturation and rather limited growth of the nanosized particles. Spectroscopic characterizations indicated that the nanocondensates have a significant internal compressive stress, (H+, Zr2+, Zr3+) co-signature and hence a smaller band gap of 5.2–5.3 eV for potential applications in UV region.










Similar content being viewed by others
References
Berthe L, Fabbro R, Peyre L, Tollier L, Bartnicki E (1997) Shock waves from a water-confined laser-generated plasma. J Appl Phys 82:2826–2832
Bolis V, Magnacca G, Cerrato G, Morterra C (2001) Microcalorimetric and IR spectroscopy study of the room temperature adsorption of CO2 on pure and sulphated t-ZrO2. Thermochim Acta 379:147–161
Bouvier P, Lucazeau G (2000) Raman spectra and vibrational analysis of nanometric tetragonal zirconia under high pressure. J Phys Chem Solids 61:569–578
Chen L, Mashimo T, Omurzak E, Okudera H, Iwamoto C, Yoshiasa A (2011) Pure tetragonal ZrO2 nanoparticles synthesized by pulsed plasma in liquid. J Phys Chem C 115:9370–9375
Chraska T, King AH, Berndt CC (2000) On the size-dependent phase transformation in nanoparticulate zirconia. Mater Sci Eng A 286:169–178
Ciuparu D, Ensuque A, Shafeev G, Bozon-Verduraz F (2000) Synthesis and apparent bandgap of nanophase zirconia. J Mater Sci Lett 19:931–933
Dupin JC, Gonbeau D, Vinatier P, Levasseur A (2000) Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys Chem Chem Phys 2:1319–1324
Fabbro R, Fournier J, Ballard P, Devaux D, Virmont J (1990) Physical study of laser-produced plasma in confined geometry. J Appl Phys 68:775–784
Fabris S, Paxton AT, Finnis MW (2002) A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater 50:5171–5178
Garvie RC (1965) The occurrence of metastable tetragonal zirconia as crystallite size effect. J Phys Chem 69:1238–1243
Giese RF Jr (1976) Hydroxyl orientations in gibbsite and bayerite. Acta Cryst B 32:1719–1723
Green DJ, Hannink RHJ, Swain MV (1989) Transformation toughening of ceramics. Boca Raton, CRC Press
Guo X (1999) On the degradation of zirconia ceramics during low-temperature annealing in water or water vapor. J Phys Chem Solids 60:539–546
Holland TJB, Redfern SAT (1997a) UNITCELL: a nonlinear least-squares program for cell-parameter refinement and implementing regression and deletion diagnostics. J Appl Cryst 30:84
Holland TJB, Redfern SAT (1997b) Unit cell refinement from powder diffraction data: the use of regression diagnostics. Mineral Mag 61:65–77
Kontoyannis CG, Orkoula M (1994) Quantitative determination of the cubic, tetragonal and monoclinic phases in partially stabilized zirconias by Raman spectroscopy. J Mater Sci 29:5316–5320
Králik B, Chang EK, Louie SG (1998) Structural properties and quasiparticle band structure of zirconia. Phys Rev B 57:7027–7036
Li M, Feng Z, Xiong G, Ying P, Xin Q, Li C (2001) Phase transformation in the surface region of zirconia detected by UV Raman spectroscopy. J Phys Chem B 105:8107–8111
Lieth RMA (1977) Preparation and crystal growth of materials with layered structures. Kluwer Academic Publishers, D. Reidel Publishing Company, Holland
Lin CH, Chen SY, Ho NJ, Gan D, Shen P (2009) Shape dependent local internal stress of α-Cr2O3 nanocrystal fabricated by pulsed laser ablation. J Phys Chem Solids 70:1505–1510
Liu LG, Bassett WA (1986) Elements, oxides, and silicates: high pressure phases with implications for the Earth’s interior. Oxford University Press, New York
Michel D, Perez y Jorba M, Collongues R (1976) Study by Raman spectroscopy of order–disorder phenomena occurring in some binary oxides with fluorite-related structures. J Raman Spectrosc 5:163–180
Mitsuhashi T, Ichihara M, Tatsuke U (1974) Characterization and stabilization of metastable tetragonal ZrO2. J Am Ceram Soc 57:97–101
Morant C, Sanz JM, Galan L, Soriano L, Rueda F (1989) An XPS study of the interaction of oxygen with zirconium. Surf Sci 218:331–345
Okasaka K, Nasu H, Kamiya K (1991) Investigation of coordination state of Zr4+ ions in the sol-gel-derived ZrO2–SiO2 glasses by EXAFS. J Noncryst Solids 136:103–110
Phillippi CM, Mazdiyasni KS (1971) Infrared and Raman spectra of zirconia polymorphs. J Am Ceram Soc 54:254–258
Porter DA, Easterling KE, Sherif MY (2009) Phase transformations in metals and alloys, 3rd edn. CRC Press, Boca Raton
Quyang F, Yao S (2000) Infrared study of ZrO2 surface sites using adsorbed probe molecules. 2. dimethyl ether adsorption. J Phys Chem B 104:11253–11257
Shen P, Lee WH (2001) (111)-specific coalescence twinning and martensitic transformation of tetragonal ZrO2 condensates. Nano Lett 1:707–711
Shi L, Tin KC, Wong NB (1999) Thermal stability of zirconia membranes. J Mater Sci 34:3367–3374
Shukla S, Seal S (2005) Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia. Int Mater Rev 50:45–64
Simeone D, Baldinozzi G, Gosset D, LeCaer S, Mazerolles L (2004) Impact of radiation defects on the structural stability of pure zirconia. Phys Rev B 70:134116-1–134116-7
Stefanic G, Music S, Grzeta B, Popovic S, Sekulic A (1998) Influence of pH on the stability of low temperature t-ZrO2. J Phys Chem Solids 59:879–885
Tan D, Teng Y, Liu Y, Zhuang Y, Qiu J (2009) Preparation of zirconia nanoparticles by pulsed laser ablation in liquid. Chem Lett 38:1102–1103
Teufer G (1962) The crystal structure of tetragonal ZrO2. Acta Cryst 15:1187
Tsai MH, Chen SY, Shen P (2005) Condensation and relaxation/transformation of dense t-ZrO2 nanoparticles. J Chem Phys 122:204708-1–204708-6
Tsai MH, Chen SY, Shen P (2006) Laser ablation condensation of polymorphic ZrO2 nanoparticles: effects of laser parameters, residual stress, and kinetic phase change. J Appl Phys 99:054302-1–054302-8
Wefers K, Misra C (1987) Oxides and hydroxides of aluminum. Alcoa Technical Paper No. 19, Revised. Alcoa Laboratories, Pittsburgh
Whitney ED (1965) Electrical resistivity and diffusionless phase transformations of zirconia at high temperatures and ultrahigh pressures. J Electrochem Soc 112:91–94
Acknowledgements
The authors thank Miss S.Y. Shih for the help on XPS analysis and anonymous referees for constructive comments. This study was supported by Center for Nanoscience and Nanotechnology at NSYSU and National Science Council, Taiwan, ROC.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
a TEM lattice image of the c-ZrO2 nanoparticles fabricated by PLAL at 700 mJ/pulse for 5 min and then subject to electron dosage for 5 min (b, c) and d, e 2D Fourier forward and inverse Fourier transform from the square regions I and II, respectively in (a) showing dislocation with (1\( \overline{1} \)1) half plane and (1\( \overline{1} \)1) fault due to a coalescence event
Hypothetical free energy versus specific volume curves of c-, t-, and m-ZrO2 phases for polymorphic phase transformations during PLA. Note the equilibrium t/m phase boundary was believed to follow a negative dT/dP slope up to 1.5 GPa (Whitney 1965). The c/t phase boundary is also expected to be negative since the c-fluorite structure is more closely packed than the t form (Liu and Bassett 1986). Thus, from thermodynamic point of view, zirconia should be c-, t-, and m-phase in the order of increasing specific volume having 2-phase equilibria defined by the cotangent of the free energy versus cell volume curves. However kinetic phase transition to form metastable intermediate of ZrO2 (solid arrows) may still occur at a specified cell volume depending on the intersection of the free energy versus cell volume curves (i.e., V o or V o′) under the influence of temperature and size (i.e., capillarity effect) as indicated by previous thermodynamic calculations on c- and t-forms (Tsai et al. 2006) and the hypothetical curve of m-phase here
Rights and permissions
About this article
Cite this article
Wu, CH., Huang, CN., Shen, P. et al. Surface modification, martensitic transformation, and optical properties of hydrogenated ZrO2 nanocondensates via pulsed laser ablation in water. J Nanopart Res 13, 6633–6648 (2011). https://doi.org/10.1007/s11051-011-0571-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-011-0571-0