Abstract
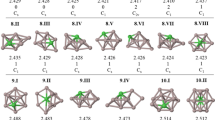
Low-lying equilibrium geometric structures of Phosphorus-doped aluminum cluster Al n P (n = 2–12) clusters obtained by an all-electron linear combination of atomic orbital approach, within spin-polarized density functional theory, are reported. The binding energy, dissociation energy, and stability of these clusters are studied within the local spin density approximation (LSDA) and the three-parameter hybrid generalized gradient approximation (GGA) due to Becke-Lee-Yang-Parr (B3LYP). Ionization potentials, electron affinities, hardness, and static polarizabilities are calculated for the ground-state structures within the GGA. It is observed that symmetric structures with the P atom occupying a peripheral position are lowest-energy geometries of Al n P (n = 2, 4–11), while the P impurities of Al3P and Al12P prefer to occupy internal sites in the aluminum clusters. Generalized gradient approximation extends bond lengths as compared to the LSDA lengths. The odd-even oscillations in the dissociation energy, the second differences in energy, the HOMO–LUMO gaps, the ionization potential, the electron affinity, and the hardness are more pronounced within both GGA and LSDA. The stability analysis based on the energies clearly shows the clusters with an even number of valence electrons are more stable than clusters with odd number of valence electrons.








Similar content being viewed by others
References
Akola J, Hakkinen H, Manninen M (1998) Ionization potential of aluminum clusters. Phys Rev B 58(7):3601–3604
Akola J, Manninen M, Hakkinen H, Landman U, Li X, Wang LS (2000) Aluminum cluster anions: photoelectron spectroscopy and ab initio simulations. Phys Rev B 62(19):13216–13228
Akutsu M, Koyasu K, Atobe J, Hosoya N, Miyajima K, Mitsui M, Nakajima A (2006) Experimental and theoretical characterization of aluminum-based binary superatoms of Al12X and their cluster salts. J Phys Chem A 110(44):12073–12076
Archibong EF, St-Amant A (2002) Structure and electron detachment energies of Al3P− and Al3P −3 . J Phys Chem A 106(24):5932–5937
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098–3100
Ceperley MD, Alder B (1980) Ground state of the electron gas by a stochastic method. J Phys Rev Lett 45(7):566–569
Deshpande MD, Kanhere DG, Vasiliev I, Martin RM (2003) Ab initio absorption spectra of Aln (n = 2–13) clusters. Phys Rev B 68(3):035428–035432
Dhavale A, Kanhere DG (2002) Density-functional investigation of the size dependence of the electronic structure of mixed aluminum-sodium clusters. Phys Rev B 65(8):085402
Feng PY, Balasubramanian K (1999) Electronic states of Al3P and AlP3 and their positive ions. Chem Phys Lett 301(5):458–466
Feng PY, Balasubramanian K (2000) Potential energy surfaces of electronic states of AlP2, Al2P and their ions. Chem Phys Lett 318(5):417–426
Francl MM, Petro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pole JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77(7):3654–3665
Frisch MJ, Trucks GW, Schlegel HB, et al., Computer code GAUSSIAN98, revision A.6 (Gaussian, Inc., Pittsburgh, PA, 1998)
Gantefor G, Meiwes-Broer KH, Lutz HO (1988) Photodetachment spectroscopy of cold aluminum cluster anions. Phys Rev A 37(7):2716–2718
Gomez H, Taylor TR, Neumark DM (2001) Anion photoelectron spectroscopy of aluminum phosphide clusters. J Phys Chem A 105(28):6886–6893
Guo L, Wu H, Jin Z (2004) First principles study of the structure, electronic state and stability of AlnP +m cations. J Mol Struct (THEOCHEM) 680(2):121–126
Guo L, Wu H, Jin Z (2005) The aluminum phosphides Al m P n (m + n = 2–5) and their anions: structures, electron affinities and vibrational frequencies. Int J Mass Spectrom 240(2):149–159
Hanley L, Ruatta SA, Anderson SL (1987) Collision-induced dissociation of aluminum cluster ions: fragmentation patterns, bond energies, and structures for Al +2 –Al +7 . J Chem Phys 87(1):260–268
Jaque P, Toro-Labbe A (2002) Characterization of copper clusters through the use of density functional theory reactivity descriptors. J Chem Phys 117(7):3208–3218
Jarrold MF, Bower JE, Kraus JS (1987) Collison induced dissociation of metal cluster ions: bare aluminum clusters, Al +n (3–26). J Chem Phys 86(7):3876–3885
Jones RO, Gunnarsson O (1989) The density functional formalism, its applications and prospects. Rev Mod Phys 61(3):689–746
Jones RO (1993) Simulated annealing study of neutral and charged clusters: Aln and Gan. J Chem Phys 99(2):1194–1206
Khanna SN, Ashman C, Rao BK, Jena P (2001) Geometry, electronic structure, and energetic of copper-doped aluminum clusters. J Chem Phys 114(8):9792–9796
Knight WD, Clemenger K, De Heer WA, Saunders WA, Chou MY, Cohen L (1984) Electronic shell structure and abundances of sodium clusters. Phys Rev Lett 52(24):2141–2143
Kumar V, Bhattacharjee S, Kawazoe Y (2000) Silicon-doped icosahedral, cuboctahedral, and decahedral clusters of aluminum. Phys Rev B 61(12):8541–8547
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789
Leuchtner RE, Harms AC, Castleman AW Jr (1991) Aluminum cluster reaction. J Chem Phys 94(2):1093–1101
Liu ZY, Wang CR, Huang RB, Zheng LS (1995) Mass distributions of binary aluminum cluster anions AlnX −m (X = O, S, P, As, C). Int J Mass Spectrom 141(3):201–208
Majumder C, Das GP, Kulshrestha SK, Shah V, Kanhere DG (1996) Ground state geometries and energetics of ALnLi (n = 1, 13) clusters using ab initio density-based molecular dynamics. Chem Phys Lett 261(5):515–520
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105(26):7512–7516
Parr RG, Chattaraj PK (1991) Principle of maximum hardness. J Am Chem Soc 113(5):1854–1855
Parr RG, Yang W (1989) Density functional theory of atoms, molecules. Oxford, New York
Pearson RG (1997) Chemical hardness: applications from molecules to solids. Wiley-VCH, Weinheim
Petterson LGM, Bauschlicher CW Jr (1987) Small Al clusters. II. Structure and binding in Aln (n = 2–6, 13). J Chem Phys 87(4):2205–2213
Rao BK, Jena P (1999) Evolution of the electronic structure and properties of neutral and charged aluminum clusters: a comprehensive analysis. J Chem Phys 111(5):1890–1904
Rao BK, Jena P (2001) Energetic and electronic structure of carbon doped aluminum clusters. J Chem Phys 115(2):778–783
Rothlisberger U, Andreoni W (1992) Structural and electronic properties of sodium microclusters (n = 2–20) at low and high temperatures: new insights from ab initio molecular dynamics studies. J Chem Phys 94(12):8129–8151
Saunders WA, Fayet P, Woste L (1989) Photodestruction of positively and negatively charged aluminum-cluster ions. Phys Rev A 39(9):4400–4405
Thomas OC, Zheng WJ, Lippa TP, Xu SJ, Lyapustina SA (2001) In search of theoretically predicted magic clusters: lithium-doped aluminum cluster anions. J Chem Phys 114(8):9895–9900
Turner GW, Johnston RL, Wilso NT (2000) Investigation of geometric shell aluminum clusters using the gupta many-body potential. J Chem Phys 112(10):4773–4778
Upton TH (1987) Aperturbed electron droplet model for the electronic structure of small aluminum clusters. J Chem Phys 86(12):7054–7064
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20603021), Youth Foundation of Shanxi (2007021009) and the Youth Academic Leader of Shanxi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, L., Wu, H. Density functional study of structural and electronic properties of Al n P (2 ≤ n ≤ 12) clusters. J Nanopart Res 10, 341–351 (2008). https://doi.org/10.1007/s11051-007-9258-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-007-9258-y