Abstract
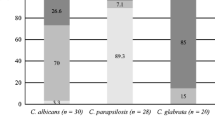
Candida spp. biofilm is considered highly resistant to conventional antifungals. The aim of this study was to investigate the in vitro effect of amphotericin B on Candida spp. biofilms at different stages of maturation. We investigated the activity of amphotericin B against 78 clinical isolates of Candida spp., representing three species, growing as planktonic and sessile cells, by a widely accepted broth microdilution method. The in vitro effect on sessile cell viability was evaluated by MTT reduction assay. All examined strains were susceptible to amphotericin B when grown as free-living cells. At the early stages of biofilm maturation 96.7–100.0 % strains, depending on species, displayed amphotericin B sessile minimal inhibitory concentration (SMIC) ≤1 μg/mL. Mature Candida spp. biofilm of 32.1–90.0 % strains displayed amphotericin B SMIC ≤1 μg/mL. Based on these results, amphotericin B displays species- and strain-depending activity against Candida spp. biofilms.
Similar content being viewed by others
References
Costerton J, Stewart PS, Greenberg E. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22.
Harriott MM, Lilly EA, Rodriguez TE, et al. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156:3635–44.
Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–67.
Ramage G, Saville SP, Thomas DP, et al. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–8.
Salerno C, Pascale M, Contaldo M, et al. Candida-associated denture stomatitis. Med Oral Patol Oral Cir Bucal. 2011;16:e139–43.
Seneviratne CJ, Silva WJ, Jin LJ, et al. Architectural analysis, viability assessment and growth kinetics of Candida albicans and Candida glabrata biofilms. Arch Oral Biol. 2009;54:1052–60.
Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342.
Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2003;38:161–89.
Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55:999–1008.
Baillie GS, Douglas LJ. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 1999;310:644–56.
Chandra J, Kuhn DM, Mukherjee PK, et al. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–94.
Chandra J, Mukherjee PK, Leidich SD, et al. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80:903–8.
Choi HW, Shin JH, Jung SI, et al. Species-specific differences in the susceptibilities of biofilms formed by Candida bloodstream isolates to echinocandin antifungals. Antimicrob Agents Chemother. 2007;51:1520–3.
Hawser SP, Douglas LJ. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 1995;39:2128–31.
Kuhn DM, George T, Chandra J, et al. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–80.
Ramage G, VandeWalle K, Wickes BL, et al. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–9.
Růzicka F, Holá V, Votava M, et al. Importance of biofilm in Candida parapsilosis and evaluation of its susceptibility to antifungal agents by colorimetric method. Folia Microbiol. 2007;52:209–14.
Shuford JA, Piper KE, Steckelberg JM, et al. In vitro biofilm characterization and activity of antifungal agents alone and in combination against sessile and planktonic clinical Candida albicans isolates. Diagn Microbiol Infect Dis. 2007;57:277–81.
Shuford JA, Rouse MS, Piper KE, et al. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J Infect Dis. 2006;194:710–3.
Baginski M, Czub J. Amphotericin B and its new derivatives—mode of action. Curr Drug Metab. 2009;10:459–69.
Boyd RF, Hoerl BG. Laboratory manual to accompany basic medical microbiology. 2nd ed. Boston: Little, Brown and Company; 1981.
Deak T, Beuchat LR. Comparison of the SIM, API 20 C and ID 32 C systems for indentification of yeasts isolated from juice concentrates and beverages. J Food Prot. 1993;56:585–92.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts—third edition: approved standard M27-A3. Wayne, PA: CLSI; 2008.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts—third informational supplement M27-S3. Wayne, PA: CLSI; 2008.
Ramage G, López-Ribot JL. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol Med. 2005;18:71–91.
Krom BP, Cohen JB, McElhaney-Feser G, et al. Conditions for optimal Candida biofilm development in microtiter plates. Methods Mol Biol. 2009;499:55–62.
Polak A. Pharmacokinetics of amphotericin B and flucytosine. Postgrad Med J. 1979;55:667–70.
Silva S, Henriques M, Martins A, et al. Biofilm of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol. 2009;47:681–9.
Jabra-Rizk MA, Falkler WA, Meiller TF. Fungal biofilms and drug resistance. EID. 2004;10:14–9.
Kuhn DM, Chandra J, Mukherjee PK, et al. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70:878–88.
Pfaller MA, Messer SA, Hollis RJ. Variations in DNA subtype, antifungal susceptibility, and slime production among clinical isolates of Candida parapsilosis. Diagn Microbiol Infect Dis. 1995;21:9–14.
Acknowledgments
This work was supported by the Nicolaus Copernicus University in Toruń, Ludwik Rydygier Collegium Medicum in Bydgoszcz (Grant Number 06/CM).
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prażyńska, M., Gospodarek, E. In Vitro Effect of Amphotericin B on Candida albicans, Candida glabrata and Candida parapsilosis Biofilm Formation. Mycopathologia 177, 19–27 (2014). https://doi.org/10.1007/s11046-014-9727-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-014-9727-7