Abstract
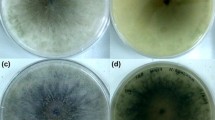
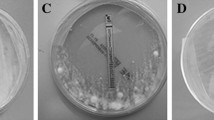
Trichophyton rubrum, an anthropophilic dermatophyte fungus, is the predominant causative agent of superficial skin infections in human population. There are only scanty reports on drug susceptibility profiling of T. rubrum. Neither mechanisms for drug resistance development nor correlation between in vitro drug susceptibility and in vivo response to treatment is known for that species. In this study, changes in the in vitro susceptibilities to fluconazole (FLZ) and itraconazole (ITZ) among thirty T. rubrum clinical strains subjected to sequential passages in the presence or absence of the azoles were investigated. Each strain was passaged 12 times at 4-week intervals as three parallel cultures, maintained on a drug-free medium (1), and a medium containing FLZ (2) or ITZ (3) at subinhibitory concentrations. Susceptibility to FLZ and ITZ of the original strain and its 3 subcultures was determined by microdilution method. The MIC values of the two azoles remained unaltered for all T. rubrum strains tested, after 12 passages on a drug-free medium. Among the strains grown with FLZ, an increase in the MICs of FLZ and ITZ was noted in 17 (56.7 %) and 19 (63.3 %) strains, respectively. Increased MICs of ITZ and FLZ were demonstrated for 24 (80 %) and 20 (66.7 %) strains that were propagated with ITZ. The results indicate the capacity of T. rubrum to develop resistance toward the azoles after prolonged exposure to these drugs. Resistance of T. rubrum to azoles plays an important role in therapy failures and consequently contributes to persistence and chronicity of the infections.

Similar content being viewed by others
References
Aly R. Ecology and epidemiology of dermatophyte infections. J Am Acad Dermatol. 1994;31(Suppl 2):21–5.
Tietz HJ, Kunzelmann V, Schönian G. Changes in the fungal spectrum of dermatomycoses. Mycoses. 1995;38:33–9.
Burzykowski T, Molenberghs G, Abeck D, et al. High prevalence of foot diseases in Europe: results of the Achilles Project. Mycoses. 2003;46:496–505.
Lacroix C, Baspeyras M, de La Salmonière P, et al. Tinea pedis in European marathon runners. J Eur Acad Dermatol Venereol. 2002;16:139–42.
Gentles JC, Holmes JG. Foot ringworm in coal-miners. Br J Ind Med. 1957;3:22–9.
Noguchi H, Hiruma M, Kawada A, Ishibashi A, Kono S. Tinea pedis in members of the Japanese Self-defence Forces: relationships of its prevalence and its severity with length of military service and width of interdigital spaces. Mycoses. 1995;38:494–9.
Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–17.
Szepietowski JC, Reich A. Stigmatisation in onychomycosis patients: a population-based study. Mycoses. 2009;52:343–9.
Drake LA, Patrick DL, Fleckman P, et al. The impact of onychomycosis on quality of life: development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol. 1999;41:189–96.
Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754–6.
Cowen LE, Sanglard D, Calabrese D, Sirjusingh C, Anderson JB, Kohn LM. Evolution of drug resistance in experimental populations of Candida albicans. J Bacteriol. 2000;182:1515–22.
Arikan S. Current status of antifungal susceptibility testing methods. Med Mycol. 2007;45:569–87.
Hryncewicz-Gwóźdź A, Jagielski T, Sadakierska-Chudy A, Dyląg M, Pawlik K, Baran E, et al. Molecular typing of Trichophyton rubrum clinical isolates from Poland. Mycoses. 2011;54:726–36.
Santos AD, de Carvalho Araújo RA, Santos AD, Kohler LM. Molecular typing and antifungal susceptibility of Trichophyton rubrum isolates from patients with onychomycosis pre- and post-treatment. Int J Antimicrob Agents. 2007;29:563–9.
Fachin AL, Maffei CM, Martinez-Rossi NM. In vitro susceptibility of Trichophyton rubrum isolates to griseofulvin and tioconazole. Induction and isolation of a resistant mutant to both antimycotic drugs. Mutant of Trichophyton rubrum resistant to griseofulvin and tioconazole. Mycopathologia. 1996;135:141–3.
Ghannoum MA, Arthington-Skaggs B, Chaturvedi V, et al. Interlaboratory study of quality control isolates for a broth microdilution method (modified CLSI M38-A) for testing susceptibilities of dermatophytes to antifungals. J Clin Microbiol. 2006;44:4353–6.
Barros da Silva ME, de Assis Santos D, Hamdan JS. Evaluation of susceptibility of Trichophyton mentagrophytes and Trichophyton rubrum clinical isolates to antifungal drugs using a modified CLSI microdilution method (M38-A). J Med Microbiol. 2007;56:514–8.
Osborne CS, Hofbauer B, Favre B, Ryder NS. In vitro analysis of the ability of Trichophyton rubrum to become resistant to terbinafine. Antimicrob Agents Chemother. 2003;47:3634–6.
Barchiesi F, Calabrese D, Sanglard D, et al. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob Agents Chemother. 2000;44:1578–84.
Marr KA, Lyons CN, Ha K, Rustad TR, White TC. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob Agents Chemother. 2001;45:52–9.
Seo K, Akiyoshi H, Ohnishi Y. Alteration of cell wall composition leads to amphotericin B resistance in Aspergillus flavus. Microbiol Immunol. 1999;43:1017–25.
Manavathu EK, Vazquez JA, Chandrasekar PH. Reduced susceptibility in laboratory-selected mutants of Aspergillus fumigatus to itraconazole due to decreased intracellular accumulation of the antifungal agent. Int J Antimicrob Agents. 1999;12:213–9.
Gupta AK, Kohli Y. Evaluation of in vitro resistance in patients with onychomycosis who fail antifungal therapy. Dermatology. 2003;207:375–80.
Martinez-Rossi NM, Peres NTA, Rossi A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia. 2008;166:369–83.
Fernández-Torres B, Carrillo AJ, Martìn E, et al. In vitro activities of 10 antifungal drugs against 508 dermatophyte strains. Antimicrob Agents Chemother. 2001;45:2524–8.
Marr KA, Lyons CN, Ha K, Rustad TR, White TC. Inducible azole resistance associated with a heterogeneous phenotype in Candida albicans. Antimicrob Agents Chemother. 2001;45:52–9.
Yamazumi T, Pfaller MA, Messer SA, et al. Characterization of heteroresistance to fluconazole among clinical isolates of Cryptococcus neoformans. J Clin Microbiol. 2003;41:267–72.
Hryncewicz-Gwóźdź A, Jagielski T, Kalinowska K, Baczynska D, Plomer-Niezgoda E, Bielecki J. Stability of tandemly repetitive subelement PCR patterns in Trichophyton rubrum over serial passaging and with respect to drug pressure. Mycopathologia. 2012;174:383–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hryncewicz-Gwóźdź, A., Kalinowska, K., Plomer-Niezgoda, E. et al. Increase in Resistance to Fluconazole and Itraconazole in Trichophyton rubrum Clinical Isolates by Sequential Passages In Vitro under Drug Pressure. Mycopathologia 176, 49–55 (2013). https://doi.org/10.1007/s11046-013-9655-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-013-9655-y