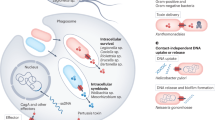
Abstract
Secretion pathways in fungi are essential for the maintenance of cell wall architecture and for the export of a number of virulence factors. In the fungal pathogen, Cryptococcus neoformans, much evidence supports the existence of more than one route taken by secreted molecules to reach the cell periphery and extracellular space, and a significant degree of crosstalk between conventional and non-conventional secretion routes. The need for such complexity may be due to differences in the nature of the exported cargo, the spatial and temporal requirements for constitutive and non-constitutive protein secretion, and/or as a means of compensating for the extra burden on the secretion machinery imposed by the elaboration of the polysaccharide capsule. This review focuses on the role of specific components of the C. neoformans secretion machinery in protein and/or polysaccharide export, including Sec4, Sec6, Sec14, Golgi reassembly and stacking protein and extracellular exosome-like vesicles. We also address what is known about traffic of the lipid, glucosylceramide, a target of therapeutic antibodies and an important regulator of C. neoformans pathogenicity, and the role of signalling pathways in the regulation of secretion.
Similar content being viewed by others
References
Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009;68:133–216. doi:10.1016/S0065-2164(09)01204-0.
Rodrigues ML, Casadevall A, Zaragoza O. The architecture and antigenic composition of the polysaccharide capsule. In: Joseph Heitman TK, Kwon-Chung J, Perfect J, Casadevall A, editors. Cryptococcus: from human pathogen to model yeast. American Society for Microbiology; 2011. p. 43–54.
Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68(2):443–8.
Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, et al. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol. 2011;80(4):1088–101. doi:10.1111/j.1365-2958.2011.07632.x.
Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39(1):166–75. doi:10.1046/j.1365-2958.2001.02236.x.
Djordjevic JT, Del Poeta M, Sorrell TC, Turner KM, Wright LC. Secretion of cryptococcal phospholipase B1 (PLB1) is regulated by a glycosylphosphatidylinositol (GPI) anchor. Biochem J. 2005;389(Pt 3):803–12. doi:10.1042/BJ20050063.
Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155(Pt 12):3860–7. doi:10.1099/mic.0.032854-0.
Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, et al. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol Microbiol. 2009;71(5):1165–76. doi:10.1111/j.1365-2958.2008.06588.x.
Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184(2):377–86.
Williamson PR. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front Biosci. 1997;2:e99–107.
Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006;6(4):513–24. doi:10.1111/j.1567-1364.2006.00071.x.
Eigenheer RA, Jin Lee Y, Blumwald E, Phinney BS, Gelli A. Extracellular glycosylphosphatidylinositol-anchored mannoproteins and proteases of Cryptococcus neoformans. FEMS Yeast Res. 2007;7(4):499–510. doi:10.1111/j.1567-1364.2006.00198.x.
Reese AJ, Doering TL. Cell wall alpha-1, 3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol Microbiol. 2003;50(4):1401–9. doi:10.1046/j.1365-2958.2003.03780.x.
Gilbert NM, Donlin MJ, Gerik KJ, Specht CA, Djordjevic JT, Wilson CF, et al. KRE genes are required for beta-1, 6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in Cryptococcus neoformans. Mol Microbiol. 2010;76(2):517–34. doi:10.1111/j.1365-2958.2010.07119.x.
Hruskova-Heidingsfeldova O. Secreted proteins of Candida albicans. Front Biosci. 2008;13:7227–42. doi:10.2741/3224.
Schekman R. Lasker basic medical research award. SEC mutants and the secretory apparatus. Nat Med. 2002;8(10):1055–8. doi:10.1038/nm769.
Kmetzsch L, Joffe LS, Staats CC, de Oliveira DL, Fonseca FL, Cordero RJ, et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol. 2011;81:206–18. doi:10.1111/j.1365-2958.2011.07686.x.
Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6(1):48–59. doi:10.1128/EC.00318-06.
Yoneda A, Doering TL. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol Biol Cell. 2006;17(12):5131–40. doi:10.1091/mbc.E06-08-0701.
Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109(1):61–72.
Mehul B, Hughes RC. Plasma membrane targeting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J Cell Sci. 1997;110(Pt 10):1169–78.
Muesch A, Hartmann E, Rohde K, Rubartelli A, Sitia R, Rapoport TA. A novel pathway for secretory proteins? Trends Biochem Sci. 1990;15(3):86–8.
Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267(34):24161–4.
Rubartelli A, Bajetto A, Bonifaci N, Di Blas E, Solito E, Sitia R. A novel way to get out of the cell. Cytotechnology. 1993;11(Suppl 1):S37–40.
Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11(12):1421–31.
Rubartelli A, Sitia R. Interleukin 1 beta and thioredoxin are secreted through a novel pathway of secretion. Biochem Soc Trans. 1991;19(2):255–9.
Ferro-Novick S, Novick P, Field C, Schekman R. Yeast secretory mutants that block the formation of active cell surface enzymes. J Cell Biol. 1984;98(1):35–43.
Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21(1):205–15. doi:10.1016/0092-8674(80)90128-2.
Novick P, Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983;96(2):541–7.
Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76(4):1858–62.
Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108(4):1271–81.
Siafakas AR, Sorrell TC, Wright LC, Wilson C, Larsen M, Boadle R, et al. Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J Biol Chem. 2007;282(52):37508–14. doi:10.1074/jbc.M707913200.
Chen SC, Wright LC, Golding JC, Sorrell TC. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem J. 2000;347(Pt 2):431–9.
Chen SC, Wright LC, Santangelo RT, Muller M, Moran VR, Kuchel PW, et al. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect Immun. 1997;65(2):405–11.
Ganendren R, Carter E, Sorrell T, Widmer F, Wright L. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 2006;8(4):1006–15. doi:10.1016/j.micinf.2005.10.018.
Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71(3):1538–47.
Santangelo R, Zoellner H, Sorrell T, Wilson C, Donald C, Djordjevic J, et al. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect Immun. 2004;72(4):2229–39.
Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun. 2001;69(9):5589–96.
Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, et al. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell. 2005;4(1):190–201. doi:10.1128/EC.4.1.190-201.2005.
Waterman SR, Hacham M, Panepinto J, Hu G, Shin S, Williamson PR. Cell wall targeting of laccase of Cryptococcus neoformans during infection of mice. Infect Immun. 2007;75(2):714–22. doi:10.1128/IAI.01351-06.
Staib F, Grave B, Altmann L, Mishra SK, Abel T, Blisse A. Epidemiology of Cryptococcus neoformans. Mycopathologia. 1978;65(1–3):73–6.
Staib F, Mishra SK, Able T, Blisse A. Growth of Cryptococcus neoformans on uric acid agar. Zentralbl Bakteriol Orig A. 1976;236(2–3):374–85.
Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120(5):1683–93. doi:10.1172/JCI41963.
Lee IR, Chow EW, Morrow CA, Djordjevic JT, Fraser JA. Nitrogen metabolite repression of metabolism and virulence in the human fungal pathogen Cryptococcus neoformans. Genetics. 2011;188(2):309–23. doi:10.1534/genetics.111.128538.
Canteros CE, Rodero L, Rivas MC, Davel G. A rapid urease test for presumptive identification of Cryptococcus neoformans. Mycopathologia. 1996;136(1):21–3.
Hotzel H, Kielstein P, Blaschke-Hellmessen R, Wendisch J, Bar W. Phenotypic and genotypic differentiation of several human and avian isolates of Cryptococcus neoformans. Mycoses. 1998;41(9–10):389–96.
Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7(1):58–67. doi:10.1128/EC.00370-07.
Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics. 2009;9(10):2820–35. doi:10.1002/pmic.200800793.
Stahl PD, Barbieri MA. Multivesicular bodies and multivesicular endosomes: the “ins and outs” of endosomal traffic. Sci STKE. 2002;2002(141):pe32. doi:10.1126/stke.2002.141.pe32.
Siafakas AR, Wright LC, Sorrell TC, Djordjevic JT. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot Cell. 2006;5(3):488–98. doi:10.1128/EC.5.3.488-498.2006.
Turner KM, Wright LC, Sorrell TC, Djordjevic JT. N-linked glycosylation sites affect secretion of cryptococcal phospholipase B1, irrespective of glycosylphosphatidylinositol anchoring. Biochim Biophys Acta. 2006;1760(10):1569–79. doi:10.1016/j.bbagen.2006.07.002.
Djordjevic J (2011) Role of phospholipases in fungal fitness, pathogenicity and drug development-lessons from cryptococcus neoformans. Front Microbiol 1. doi:10.3389/fmicb.2010.00125.
Chayakulkeeree M, Sorrell TC, Siafakas AR, Wilson CF, Pantarat N, Gerik KJ, et al. Role and mechanism of phosphatidylinositol-specific phospholipase C in survival and virulence of Cryptococcus neoformans. Mol Microbiol. 2008;69(4):809–26. doi:10.1111/j.1365-2958.2008.06310.x.
Xie Z, Fang M, Bankaitis VA. Evidence for an intrinsic toxicity of phosphatidylcholine to Sec14p-dependent protein transport from the yeast Golgi complex. Mol Biol Cell. 2001;12(4):1117–29.
Curwin AJ, Fairn GD, McMaster CR. Phospholipid transfer protein Sec14 is required for trafficking from endosomes and regulates distinct trans-Golgi export pathways. J Biol Chem. 2009;284(11):7364–75. doi:10.1074/jbc.M808732200.
Bankaitis VA, Mousley CJ, Schaaf G. The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem Sci. 2010;35(3):150–60. doi:10.1016/j.tibs.2009.10.008.
Li X, Routt SM, Xie Z, Cui X, Fang M, Kearns MA, et al. Identification of a novel family of nonclassic yeast phosphatidylinositol transfer proteins whose function modulates phospholipase D activity and Sec14p-independent cell growth. Mol Biol Cell. 2000;11(6):1989–2005.
Monteoliva L, Sanchez M, Pla J, Gil C, Nombela C. Cloning of Candida albicans SEC14 gene homologue coding for a putative essential function. Yeast. 1996;12(11):1097–105. doi:10.1002/(SICI)1097-0061(19960915)12:11<1097:AID-YEA990>3.0.CO;2-E.
Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog. 2011;7(5):e1002047. doi:10.1371/journal.ppat.1002047.
De Jesus M, Nicola AM, Rodrigues ML, Janbon G, Casadevall A. Capsular localization of the Cryptococcus neoformans polysaccharide component galactoxylomannan. Eukaryot Cell. 2009;8(1):96–103. doi:10.1128/EC.00331-08.
Eng RH, Bishburg E, Smith SM, Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986;81(1):19–23. doi:0002-9343(86)90176-2.
Feldmesser M, Tucker S, Casadevall A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 2001;9(6):273–8. doi:S0966-842X(01)02035-2.
Salminen A, Novick PJ. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49(4):527–38. doi:0092-8674(87)90455-7.
Yoneda A, Doering TL. An unusual organelle in Cryptococcus neoformans links luminal pH and capsule biosynthesis. Fungal Genet Biol. 2009;46(9):682–7. doi:10.1016/j.fgb.2009.05.001.
Hu G, Steen BR, Lian T, Sham AP, Tam N, Tangen KL, et al. Transcriptional regulation by protein kinase A in Cryptococcus neoformans. PLoS Pathog. 2007;3(3):e42. doi:10.1371/journal.ppat.0030042.
Nickel W. Pathways of unconventional protein secretion. Curr Opin Biotechnol. 2010;21(5):621–6. doi:10.1016/j.copbio.2010.06.004.
Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10(2):148–55. doi:10.1038/nrm2617.
Cabral M, Anjard C, Malhotra V, Loomis WF, Kuspa A. Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot Cell. 2010;9(7):1009–17. doi:10.1128/EC.00337-09.
Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol. 2010;188(4):527–36. doi:10.1083/jcb.200911154.
Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 2007;130(3):524–34. doi:10.1016/j.cell.2007.06.029.
Manjithaya R, Anjard C, Loomis WF, Subramani S. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol. 2010;188(4):537–46. doi:10.1083/jcb.200911149.
Schotman H, Karhinen L, Rabouille C. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev Cell. 2008;14(2):171–82. doi:10.1016/j.devcel.2007.12.006.
Vinke FP, Grieve AG, Rabouille C. The multiple facets of the Golgi reassembly stacking proteins. Biochem J. 2011;433(3):423–33. doi:10.1042/BJ20101540.
Behnia R, Barr FA, Flanagan JJ, Barlowe C, Munro S. The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic. J Cell Biol. 2007;176(3):255–61. doi:10.1083/jcb.200607151.
Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One. 2010;5(6):e11113. doi:10.1371/journal.pone.0011113.
Kmetzsch L, Joffe LS, Staats CC, Oliveira DL, Nimrichter L, Cordero RJ, et al. Golgi reassembly and stacking protein is required for Cryptococcus neoformans virulence and polysaccharide secretion. In: 8th international conference on cryptococcus and cryptococcosis. Charleston, USA; 2011. p. 87.
Cabral M, Anjard C, Malhotra V, Loomis WF, Kuspa A. Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot Cell. 2010;9:1009–17. doi:10.1128/EC.00337-09.
Cox RA, Best GK. Cell wall composition of two strains of Blastomyces dermatitidis exhibiting differences in virulence for mice. Infect Immun. 1972;5(4):449–53.
Schneider EF, Barran LR, Wood PJ, Siddiqui IR. Cell wall of Fusarium sulphureum. II. Chemical composition of the conidial and chlamydospore walls. Can J Microbiol. 1977;23(6):763–9.
Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75(10):4792–8. doi:10.1128/IAI.00587-07.
Rhome R, Singh A, Kechichian T, Drago M, Morace G, Luberto C, et al. Surface localization of glucosylceramide during Cryptococcus neoformans infection allows targeting as a potential antifungal. PLoS One. 2011;6(1):e15572. doi:10.1371/journal.pone.0015572.
Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH Jr, Hennig M, Luberto C, et al. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest. 2006;116(6):1651–9. doi:10.1172/JCI27890.
Rodrigues ML, Shi L, Barreto-Bergter E, Nimrichter L, Farias SE, Rodrigues EG, et al. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccin Immunol. 2007;14(10):1372–6. doi:10.1128/CVI.00202-07.
Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun. 2000;68(12):7049–60.
Nimrichter L, Rodrigues ML, Rodrigues EG, Travassos LR. The multitude of targets for the immune system and drug therapy in the fungal cell wall. Microbes Infect. 2005;7(4):789–98. doi:10.1016/j.micinf.2005.03.002.
Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, et al. Biogenesis of extracellular vesicles in yeast: many questions with few answers. Commun Integr Biol. 2010;3(6):533–5. doi:10.4161/cib.3.6.12756.
Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17(4):158–62. doi:10.1016/j.tim.2008.12.005.
Nosanchuk JD, Nimrichter L, Casadevall A, Rodrigues ML. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun Integr Biol. 2008;1(1):37–9.
Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. Vesicular trans-cell wall transport in fungi: a mechanism for the delivery of virulence-associated macromolecules? Lipid Insights. 2008;2:27–40.
Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147(Pt 8):2355–65.
Garcia-Rivera J, Chang YC, Kwon-Chung KJ, Casadevall A. Cryptococcus neoformans CAP59 (or Cap59p) is involved in the extracellular trafficking of capsular glucuronoxylomannan. Eukaryot Cell. 2004;3(2):385–92.
Oliveira DL, Nimrichter L, Miranda K, Frases S, Faull KF, Casadevall A, et al. Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan. Fungal Genet Biol. 2009;46(12):956–63. doi:10.1016/j.fgb.2009.09.001.
Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10(8):1695–710. doi:10.1111/j.1462-5822.2008.01160.x.
Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot Cell. 2010;10(3):343–51. doi:10.1128/EC.00227-10.
Wolf JM, Casadevall A. Extracellular Cryptococcus neoformans vesicles are stable after isolation but not in serum. In: 8th international conference on cryptococcus and cryptococcosis. Charleston, USA: Medical University of South Carolina; 2011. p. 34.
Kozubowski L, Lee SC, Heitman J. Signalling pathways in the pathogenesis of Cryptococcus. Cell Microbiol. 2009;11(3):370–80. doi:10.1111/j.1462-5822.2008.01273.x.
Hu G, Hacham M, Waterman SR, Panepinto J, Shin S, Liu X, et al. PI3K signaling of autophagy is required for starvation tolerance and virulence of Cryptococcus neoformans. J Clin Invest. 2008;118(3):1186–97. doi:10.1172/JCI32053.
Alspaugh JA, Perfect JR, Heitman J. Signal transduction pathways regulating differentiation and pathogenicity of Cryptococcus neoformans. Fungal Genet Biol. 1998;25(1):1–14. doi:10.1006/fgbi.1998.1079.
Pukkila-Worley R, Alspaugh JA. Cyclic AMP signaling in Cryptococcus neoformans. FEMS Yeast Res. 2004;4(4–5):361–7. doi:S1567135603002411.
D’Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, et al. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21(9):3179–91. doi:10.1128/MCB.21.9.3179-3191.2001.
Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16(10):2576–89. doi:10.1093/emboj/16.10.2576.
Fox DS, Djordjevic JT, Sorrel T. Signaling cascades and enzymes as Cryptococcus virulence factors In: Joseph Heitman TK, Kwon-Chung J, Perfect J, Casadevall A, editors. Cryptococcus: from human pathogen to model yeast. American Society for Microbiology; 2011. p. 217-34.
Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–47. doi:10.1146/annurev.cellbio.23.090506.123319.
Acknowledgments
MLR is supported by grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Brazil) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil). We are thankful to Professors Arturo, Casadevall, Tania Sorrell, Josh Nosanchuk, Rosana Puccia and Leonardo Nimrichter for many discussions and suggestions within the secretion field and Stephen Schibeci (Westmead Millennium Institute, University of Sydney) for proofreading this review. Work on secretion mechanisms performed in the laboratory of JTD is supported by a project grant (632634) from the National Health and Medical Research Council of Australia (NHMRC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, M.L., Djordjevic, J.T. Unravelling Secretion in Cryptococcus neoformans: More than One Way to Skin a Cat. Mycopathologia 173, 407–418 (2012). https://doi.org/10.1007/s11046-011-9468-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-011-9468-9