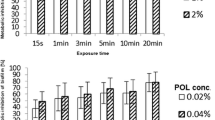
Abstract
Unlike various disinfectants, antifungals have not been commonly incorporated so far in medical devices, such as catheters or prostheses, to prevent biofilm formation by Candida spp. In the present study, five antimycotics were added to polydimethyl siloxane (PDMS) disks via admixture (nystatin) or impregnation (trimethylsilyl-nystatin (TMS-nystatin), miconazole, tea tree oil (TTO), zinc pyrithione). Nystatin-medicated PDMS disks exhibited a concentration-dependent inhibitory effect on biofilm formation in a microtiter plate (MTP) but not in a Modified Robbins Device (MRD). This observation, together with HPLC data and agar diffusion tests, indicates that a small fraction of free nystatin is released, which kills Candida albicans cells in the limited volume of a MTP well. In contrast, biofilm inhibition amounted to more than one log unit in the MRD on disks impregnated with miconazole, TTO, and zinc pyrithione. It is hypothesized that the reduction in biofilm formation by these compounds in a flow system occurs through a contact-dependent effect.

Similar content being viewed by others
References
Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17(2):255–67.
Bauters TGM, Moerman M, Vermeersch H, Nelis HJ. Colonization of voice prostheses by albicans and non-albicans Candida species. Laryngoscope. 2002;112:708–12.
Ell SR. Candida-‘the cancer of silastic’. J Laryngol Otol. 1996;110:240–2.
Arweiler-Harbeck D, Sanders A, Held M, Jerman M, Ehrich H, Jahnke K. Does metal coating improve the durability of PDMS voice prostheses? Acta Otolaryngol. 2001;121:643–6.
Dijk F, Westerhof M, Busscher HJ, van Luyn MJA, van der Mei HC. In vitro formation of oropharyngeal biofilms on PDMS rubber treated with a palladium/tin salt mixture. J Biomed Mater Res. 2000;51:408–12.
Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–9.
Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27:2450–67.
Dowling DP, Betts AJ, Pope C, McConnell ML, Eloy R, Arnaud MN. Anti-bacterial silver coatings exhibiting enhanced activity through the addition of platinum. Surf Coat Technol. 2003;163–164:637–40.
Gatter N, Kohnen W, Jansen B. In vitro efficacy of a hydrophilic central venous catheter loaded with silver to prevent microbial colonization. Zentralbl Bakteriol. 1998;287(1–2):157–69.
Kwok CS, Wan C, Hendricks S, Bryers JD, Horbett TA, Ratner BD. Design of infection-resistant antibiotic-releasing polymers. I. Fabrication and formulation. J Control Release. 1999;62:289–99.
Kwok CS, Wan C, Horbett TA, Ratner BD. Design of infection-resistant antibiotic-releasing polymers. II. Controlled release of antibiotics through a plasma-deposited thin film barrier. J Control Release. 1999;62:301–11.
Price JS, Tencer AF, Arm DM, Bohach GA. Controlled release of antibiotics from coated orthopedic implants. J Biomed Mater Res. 1996;30:281–6.
Schierholz JM. Physico-chemical properties of a rifampicin-releasing polydimethylsiloxane shunt. Biomaterials. 1997;18:635–41.
Schierholz JM, Steinhauser H, Rump AFE, Berkels R, Pulverer G. Controlled release of antibiotics from biomedical polyurethanes: morphological and structural features. Biomaterials. 1997;18:839–44.
Stigter M, Bezemer J, de Groot K, Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Control Release. 2004;99:127–37.
Von Eiff C, Kohnen W, Becker K, Jansen B. Modern strategies in the prevention of implant-associated infections. Int J Artif Organs. 2005;28(11):1146–56.
Kingston D, Seal DV, Hill ID. Self-disinfecting plastics for intravenous catheters and prosthetic inserts. J Hyg (Lond). 1986;96(2):185–98.
Darouiche RO, Mansouri MD, Kojic EM. Antifungal activity of antimicrobial-impregnated devices. Clin Microbiol Infect. 2006;12:397–9.
Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter: a randomized, controlled trial. Ann Intern Med. 1997;127:257–66.
Pigno MA, Goldschmidt MC, Lemon JC. The efficacy of antifungal agents incorporated into a facial prosthetic PDMS elastomer. J Prosthet Dent. 1994;71:295–300.
Groll AH, Mickiene D, Werner K, Piscitelli SC, Walsh TJ. High-performance liquid chromatographic determination of liposomal nystatin in plasma and tissues for pharmacokinetic and tissue distribution studies. J Chromatogr B. 1999;735:51–62.
Groll AH, Mickiene D, Werner K, Petraitiene R, Petraitis V, Calendario M, et al. Compartimental pharmacokinetics and tissue distribution of multilamellar liposomal nystatin in rabbits. Antimicrob Agents Chemother. 2000;44:950–7.
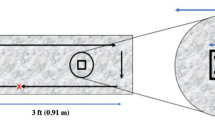
Honraet K, Nelis HJ. Use of the Modified Robbins Device, fluorescent staining to screen plant extracts for the inhibition of S. mutans biofilm formation. J Microbiol Methods. 2006;64:217–24.
Coenye T, De Prijck K, De Wever B, Nelis HJ. Use of the Modified Robbins Device to study the in vitro biofilm removal efficacy of NitrAdine™, a novel disinfecting formula for the maintenance of oral medical devices. J Appl Microbiol. 2008;105(3):733–40.
Leunisse C, van Weissenbruch R, Busscher HJ, van der Mei HC, Albers FWJ. The artificial throat: a new method for standardization of in vitro experiments with tracheo-oesophageal voice prostheses. Acta Otolaryngol. 1999;119:604–8.
De Prijck K, Nelis H, Coenye T. Efficacy of silver-releasing rubber for the prevention of Pseudomonas aeruginosa biofilm formation in water. Biofouling. 2007;23:405–11.
Acknowledgments
Funding was provided by the Belgian Federation against Cancer and FWO-Vlaanderen. The authors are indebted to Imke Hindryckx and Carole Eyi Mba for their assistance in the work. The authors are also grateful to Philomain Wauters and Mario Podevijn from the Technical Workshop of the Faculty of Science for the construction of an optimized and miniaturized version of the MRD. We are also thankful to Prof. Hubert Vermeersch from the Head and Neck Surgery Department of the Ghent University Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kristof De Prijck and Nele De Smet have contributed equally to this study.
Rights and permissions
About this article
Cite this article
De Prijck, K., De Smet, N., Honraet, K. et al. Inhibition of Candida albicans Biofilm Formation by Antimycotics Released from Modified Polydimethyl Siloxane. Mycopathologia 169, 167–174 (2010). https://doi.org/10.1007/s11046-009-9242-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-009-9242-4