Abstract
Background
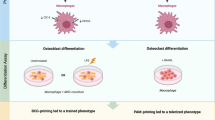
Lipoxin A4 (LXA4) is a specialized pro-resolving mediator involved in the resolution phase of inflammation that is crucial for the return of tissues to homeostasis, healing, and regenerative processes. LXA4 can modify the microenvironment via its receptor, formyl peptide receptor 2 (FPR2) and thus modulate the inflammatory response. However, the effect of exogeneous LXA4 application on polarized macrophages remains unstudied. The objective of this study was to assess the effect of LXA4 on macrophage activity and on the phenotype modulation of polarized M1 and M2 macrophages derived from THP-1 monocytes.
Methods and results
Once differentiated, human macrophages were incubated with interleukin 4 (IL-4) and IL-13 to obtain M2-polarized macrophages or with interferon gamma and lipopolysaccharide for classical macrophage activation. The mRNA and protein expression of M1 and M2 markers confirmed the polarization of THP-1-derived macrophages. LXA4 (0–100 nM) did not affect the viability of M1 and M2 macrophages or the phagocytic activity of these cells. Gene expression of FPR2, referred as a receptor for the LXA4, was higher in M1 compared with M2, and was not modified by the LXA4 at the doses used. Moreover, LXA4 exhibited anti-inflammatory properties illustrated by the decreasing in the gene expression of pro-inflammatory cytokines (IL-6, tumor necrosis factor alpha, IL-1β) in M1 and by the increase in the expression of anti-inflammatory cytokines (IL-10) in M2 macrophages.
Conclusions
These results provide new insights regarding the potential of LXA4 to regulate the polarization state of macrophages.



Similar content being viewed by others
References
Headland SE, Norling LV (2015) The resolution of inflammation: principles and challenges. Semin Immunol 27:149–160. https://doi.org/10.1016/j.smim.2015.03.014
Chen L, Deng H, Cui H et al (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9:7204–7218. https://doi.org/10.18632/oncotarget.23208
Muller WA (2002) Leukocyte-endothelial cell interactions in the inflammatory response. Lab Investig 82:521–533. https://doi.org/10.1038/labinvest.3780446
Orekhov AN, Orekhova VA, Nikiforov NG et al (2019) Monocyte differentiation and macrophage polarization. Vessel Plus. https://doi.org/10.20517/2574-1209.2019.04
Mantovani A, Biswas SK, Galdiero MR et al (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229:176–185
Ißleib C, Kurz S, Scholl S et al (2021) Plasticity of proinflammatory macrophages depends on their polarization stage during human MSC immunomodulation: an in vitro study using THP-1 and human primary macrophages. Immuno 1:518–528. https://doi.org/10.3390/immuno1040036
Yu T, Zhao L, Huang X et al (2016) Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. J Periodontol 87:1092–1102. https://doi.org/10.1902/jop.2016.160081
Zhang H, Cai D, Bai X (2020) Macrophages regulate the progression of osteoarthritis. Osteoarthr Cartil 28:555–561. https://doi.org/10.1016/j.joca.2020.01.007
Oishi Y, Manabe I (2018) Macrophages in inflammation, repair and regeneration. Int Immunol 30:511–528. https://doi.org/10.1093/intimm/dxy054
Bannenberg G, Serhan CN (2010) Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta - Mol Cell Biol Lipids 1801:1260–1273. https://doi.org/10.1016/j.bbalip.2010.08.002
Buckley CD, Gilroy DW, Serhan CN (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40:315–327
Basil MC, Levy BD (2016) Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 16:51–67. https://doi.org/10.1038/nri.2015.4
Sorgi CA, Zarini S, Martin SA et al (2017) Dormant 5-lipoxygenase in inflammatory macrophages is triggered by exogenous arachidonic acid. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-11496-3
Maciuszek M, Cacace A, Brennan E et al (2021) Recent advances in the design and development of formyl peptide receptor 2 (FPR2/ALX) agonists as pro-resolving agents with diverse therapeutic potential. Eur J Med Chem 213:113167. https://doi.org/10.1016/j.ejmech.2021.113167
Buckley CD, Gilroy DW, Serhan CN et al (2013) The resolution of inflammation. Nat Rev Immunol 13:59–66
Jaén RI, Sánchez-García S, Fernández-Velasco M et al (2021) Resolution-based therapies: the potential of lipoxins to treat human diseases. Front Immunol 12:1–14. https://doi.org/10.3389/fimmu.2021.658840
Börgeson E, Johnson AMFMF, Lee YSS et al (2015) Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab 22:125–137. https://doi.org/10.1016/j.cmet.2015.05.003
Vasconcelos DP, Costa M, Amaral IF et al (2015) Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials 37:116–123. https://doi.org/10.1016/j.biomaterials.2014.10.035
Yuan J, Lin F, Chen W, Lu H (2022) Lipoxin A4 regulates M1 / M2 macrophage polarization via FPR2-IRF pathway. Inflammopharmacology 30:1–21
Lund ME, To J, O’Brien BA, Donnelly S (2016) The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J Immunol Methods 430:64–70. https://doi.org/10.1016/j.jim.2016.01.012
Genin M, Clement F, Fattaccioli A et al (2015) M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15:1–14. https://doi.org/10.1186/s12885-015-1546-9
Jin J, Xie Y, Shi C et al (2020) Lipoxin A4 Inhibits NLRP3 Inflammasome Activation in Rats With Non-compressive Disc Herniation Through the JNK1/Beclin-1/PI3KC3 Pathway. Front Neurosci 14:799
Chandrasekharan JA, Sharma-walia N (2015) Lipoxins: nature ’s way to resolve inflammation. J Inflamm Res 8:181–192. https://doi.org/10.2147/JIR.S90380
Gaudin A, Tolar M, Peters OA (2018) Lipoxin A4 attenuates the inflammatory response in stem cells of the apical papilla via ALX/FPR2. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-27194-7
Prieto P, Cuenca J, Través PG et al (2010) Lipoxin A4 impairment of apoptotic signaling in macrophages: Implication of the PI3K/Akt and the ERK/Nrf-2 defense pathways. Cell Death Differ 17:1179–1188. https://doi.org/10.1038/cdd.2009.220
Godson C, Mitchell S, Harvey K et al (2000) Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol 164:1663–1667. https://doi.org/10.4049/jimmunol.164.4.1663
Ali M, Yang F, Jansen JA, Walboomers XF (2020) Lipoxin suppresses inflammation via the TLR4/MyD88/NF-κB pathway in periodontal ligament cells. Oral Dis 26:429–438. https://doi.org/10.1111/odi.13250
Marginean A, Sharma-Walia N (2015) Lipoxins exert antiangiogenic and anti-inflammatory effects on Kaposi’s sarcoma cells. Transl Res 166:111–133. https://doi.org/10.1016/j.trsl.2015.02.009
Nielsen MC, Gantzel RH, Cl J, et al Acute-on-Chronic Liver Failure, pp 1–18
Mussai F, De Santo C, Abu-Dayyeh I et al (2013) Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 122:749–758. https://doi.org/10.1182/blood-2013-01-480129
Dakin SG, Martinez FO, Yapp C et al (2015) Inflammation activation and resolution in human tendon disease. Sci Transl Med 7:311ra173. https://doi.org/10.1126/scitranslmed.aac4269
Jablonski KA, Amici SA, Webb LM et al (2015) Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 10:5–11. https://doi.org/10.1371/journal.pone.0145342
Park GT, Kwon YW, Lee TW et al (2019) Formyl peptide receptor 2 activation ameliorates dermal fibrosis and inflammation in bleomycin-induced scleroderma. Front Immunol 10:1–13. https://doi.org/10.3389/fimmu.2019.02095
Liu Y, Chen K, Wang C et al (2013) Cell surface receptor FPR2 promotes anti-tumor host defense by limiting M2 polarization of macrophages. Cancer Res 15:550–560. https://doi.org/10.1038/jid.2014.371
Zhuang Y, Liu H, Edward Zhou X et al (2020) Structure of formylpeptide receptor 2-Gi complex reveals insights into ligand recognition and signaling. Nat Commun 11:1–12. https://doi.org/10.1038/s41467-020-14728-9
Parisi L, Gini E, Baci D et al (2018) Macrophage polarization in chronic inflammatory diseases: killers or builders? J Immunol Res. https://doi.org/10.1155/2018/8917804
Lukic A, Larssen P, Fauland A et al (2017) GM-CSF– and M-CSF–primed macrophages present similar resolving but distinct inflammatory lipid mediator signatures. FASEB J 31:4370–4381. https://doi.org/10.1096/fj.201700319R
Rey-Giraud F, Hafner M, Ries CH (2012) In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS ONE. https://doi.org/10.1371/journal.pone.0042656
Wu J, Ding DH, Li QQ et al (2019) Lipoxin A4 regulates lipopolysaccharide-induced BV2 microglial activation and differentiation via the notch signaling pathway. Front Cell Neurosci 13:1–17. https://doi.org/10.3389/fncel.2019.00019
Cattaneo F, Parisi M, Ammendola R (2013) Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. IJMS 14:7193–7230
Xin Y, Yin M, Zhao L et al (2017) Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol Med 14:228. https://doi.org/10.20892/j.issn.2095-3941.2017.0052
Wang B, Shao J, Jansen JA et al (2019) A novel thermoresponsive gel as a potential delivery system for lipoxin. J Dent Res 98:355–362. https://doi.org/10.1177/0022034518810213
Funding
This research was funded by the Agence Nationale de la Recherche (ANR), grant number ANR-19-CE19-0006.
Author information
Authors and Affiliations
Contributions
DA, ST, AG Planned and designed the research. DA, ST, AG Wrote the manuscript. ST Performed experiments. DA, ST, AG Performed experiments and analyzed data. VG, FP Analyzed data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aubeux, D., Tessier, S., Pérez, F. et al. In vitro phenotypic effects of Lipoxin A4 on M1 and M2 polarized macrophages derived from THP-1. Mol Biol Rep 50, 339–348 (2023). https://doi.org/10.1007/s11033-022-08041-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08041-5