Abstract
Background
Soil salinity drastically reduced wheat growth and production in Pakistan. It is a need of an hour to identify the best suitable salt tolerance or resistant wheat varieties which shows good growth under salinity affected areas. In presented study, two wheat varieties Johar (salt tolerant) and Sarsabaz (salt sensitive) were examined under NaCl stress conditions.
Methods
Antioxidant enzyme activities were investigated in 10-days old wheat seedlings under 200 mM NaCl stress in hydroponic conditions. To investigate the various growth parameters, antioxidant enzyme activities such as superoxide dismutase (SOD: EC 1.15.1.1), catalase (CAT: EC 1.11.1.6) and ascorbate peroxidase (APX: EC 1.11.1.11) were monitored and studied. Besides this various growth parameters such as length of the roots, shoots, as well as Physiological parameters likes lipid peroxidation by malondialdehyde (MDA), hydrogen peroxide (H2O2), and proline contents and antioxidant enzyme activities were estimated. The effect of salinity was also observed on gene transcription level and eventually expression level.
Results
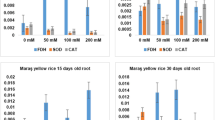
Shoot and root length were decreased in Sarsabaz variety while it showed opposite trend in johar at 200 mM salt concentration. The concentration of proline showed a noticeable rise in salt dependency. Higher concentrations of Proline in Johar were observed as compared to Sarsabaz. SOD showed the increase in activity for antioxidant enzymes. Significant increase of SOD levels were observed in shoot tissues as compared to root tissues. The results indicated that the shoots were more susceptible to salt stress. Activity of APX showed similar affects in both varieties. The production of CAT enzyme in the shoot and root tissues of both varieties showed substantial growth under increased salt stress. Furthermore, NaCl stress has increased the expression of certain genes coding for antioxidant enzymes such as catalase, superoxide dismutase, and peroxidase. Maximum expression of all the antioxidant enzyme coding genes were observed in Johar (tolerant) at 48 h exposure to salt. In contrast the expression of the all mentioned genes in Sarsabaz variety were found maximum at early hours (24 h) and gradually decreased at 48 h.
Conclusion
The study showed that the selected salt tolerant wheat variety Johar is significantly resistant to 200 mM NaCl salt level as compared to Sarsabaz.






Similar content being viewed by others
Data availability
All data are available when need.
References
Chaves MM, Pereira JS, Maroco J, Rodrigues ML (2002) How plants cope with water stress in the field: photosynthesis and growth. Ann Bot 89:907–916
Iqbal MA, Rahim J, Naeem W, Hassan S, Khattab Y, Sabagh A (2021) Rainfed winter wheat (Triticum aestivum L.) cultivars respond differently to integrated fertilization in Pakistan. Fresenius Environ Bull 30(4):3115–3121
Naheed R, Zahid M, Aqeel M et al (2022) Mediation of growth and metabolism of Pisum sativum in salt stress potentially be credited to thiamine. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00854-4
Naheed R, Zahid M, Aqeel M, Maqsood MF, Kanwal H, Khalid N, Hashem M, Alamri S, Noman A (2022) Mediation of growth and metabolism of Pisum sativumin salt stress potentially be credited to thiamine. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00854-4
Banik N, Bhattacharjee S (2020) Complementation of ROS scavenging metabolites with enzymatic antioxidant defense system augments redox-regulation property under salinity stress in rice. Physiol Mol Biol Plants 26:1623–1633
García-Caparrós P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, Altay V, Lao MT (2020) Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev 87:421
Hossain MS (2019) Present scenario of global salt affected soils, its management and importance of salinity research. Int Res J Biol Sci 1:1–3
Tufail A, Aqeel M, Khalid N, Ahsan M, Khilji SA, Ahmad F, Hameed M, Noman A, Alamri S, Hashem M (2020) Salt toxicity in a natural habitat induces structural and functional modifications and modulate metabolism in Bermuda grass (Cynodon dactylon pers.) ecotypes. Appl Ecol Environ Res 18(5):6569–6588
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Pérez-López U, Robredo A, Lacuesta M, Sgherri C, Muñoz-Rueda A, Navari-Izzo F (2009) The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol Plant 135:29–42
Zhang X, Yin HaiBo, Chen SH, He J, Guo SL (2014) Changes in antioxidant enzyme activity and transcript levels of related genes in Limonium sinense Kuntze seedlings under NaCl stress. J Chem 5(1):1–6
Rashda N, Humaira A, Hina K, Fozia F, Mohammad I, Abo G, Al-Mushhin AAM, Dilfuza J, Mohammad JA, Sehar S, Muhammad A, Ali N, Kamel H (2021) Growth attributes, biochemical modulations, antioxidant enzymatic metabolism and yield in Brassica napus varieties for salinity tolerance. Saudi J Biol Sci 28(10):5469–5479
Wani PA, Garba SH, Wahid S, Hussaini NA, Mashood KA (2019) Prevention of oxidative damage and phytoremediation of Cr (VI) by chromium (VI) reducing Bacillus subtilus PAW3 in cowpea plants. Bull Environ Contam Toxicol 103(3):476–483
Anton E, Aleksandr B, Ulyana Z, Dmitrii K, Alexey D (2019) Stress induced changes in the expression of antioxidant system genes for rice (Oriza sativa L.) and bread wheat (Triticum aestivum L.). Peer J. 29(7):1–10
Moradbeygi H, Jamei R, Heidari R, Darvishzadeh R (2020) Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci Hortic 272:109537
Moradbeygi H, Jamei R, Heidari R, Darvishzadeh R (2020) Fe2O3 nanoparticles induced biochemical responses and expression of genes involved in rosmarinic acid biosynthesis pathway in Moldavian balm under salinity stress. Physiol Plant 169:555–570
Mushtaq T, Shah AA, Akram W, Yasin NA (2020) Synergistic ameliorative effect of iron oxide nanoparticles and Bacillus subtilis S4 against arsenic toxicity in Cucurbita moschata: polyamines, anti-oxidants, and physiochemical studies. Int J Phytoremediat 22:1408–1419
Eteshami H, Hamideh F, Muhammad R (2021) Interaction of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants. Ecotoxicol Environ Safety 5(1):6654
Abdoli S, Ghassemi-Golezani K, Alizadeh-Salteh S (2020) Responses of ajowan (Trachyspermum ammi L.) to exogenous salicylic acid and iron oxide nanoparticles under salt stress. Environ Sci Pollut Res 27:36939–36953. https://doi.org/10.1007/s11356-020-09453-1
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
González A, Vidal C, Espinoza D, Moenne A (2021) Anthracene induces oxidative stress and activation of antioxidant and detoxification enzymes in Ulvalactuca (Chlorophyta). Sci Rep 11:7448
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutase in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Tatiana R, Marcelo NA, Leticia CB, Isabel LV, Eugenia JBB, Ariano MMJ, Mara AC (2017) Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiol Mol Biol Plants 23(4):856–875
Scandalios JG (2002) The rise of ROS. Trends Biochem Sci 27(9):483–486
Scandalias JG (2005) Responses of plant antioxidant defense genes to environmental stress. Adv Genet 28:1–41
Rosa SB, Caverzan A, Teixeira FK, Lazzarotto F, Silveira JA, Ferreira-Silva SL, Abreu-Neto J, Margis R, MargisPinheiro M (2010) Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71:548–558
Soraya MPB, Sedigheh FO, Ali A (2019) Expression of dehydrin and antioxidant genes and enzymatic antioxidant defense under drought stress in wild relatives of wheat. Biotech Biotec Equip 33(1):1063–1073
Zhai CZ, Zhao L, Yin LJ, Chen M, Wang QY, Li LC, Xu ZS, Ma YZ (2013) Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis. PLoS ONE 8(10):e73989
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil, 2nd edn. University of California Agricultural Experiment Station, Berkeley, Circular
Brini F, Amara I, Feki K, Hanin M, Khoudi H, Masmoudi K (2009) Physiological and molecular analyses of seedlings of two Tunisian durum wheat (Triticum turgidum L. subsp. Durum [Desf.]) varieties showing contrasting tolerance to salt stress. Acta Physiol Plant 31:145–154
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Li W, Li Q (2017) Effect of environmental salt stress on plants and the molecular mechanism of salt stress tolerance. Int J Environ Sci Nat Res. https://doi.org/10.19080/IJESNR.2017.07.555714
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Peterson RKD, Higley LG (2000) Biotic stress and yield loss, 1st edn. CRC Press, Washington, p 261
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Weimberg R (1987) Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol Plant 70:381–388
Gil R, Bautista I, Boscaiu M, LidoÂn A, Wankhade S, SaÂnchez H (2014) Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 6:1–18
Misra PS, Mertz ET, Glower DV (1975) Cereal Chem 52: 844
Moore S, Stein WH (1984). In: Colowick SP, Kaplan ND (eds) Methods in enzymol. Academic Press, New York, p 468
Beyer W, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Hernandez JA, Campillo A, Jimenez A, Alarcon JJ, Sevilla F (1999) Response of antioxidant systems and leaf water relations to NaCl stress in pea. New Phytol 141:241–251
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Klein D (2002) Quantification using real time PCR technology: applications and limitations. Trends Mol Med 8:257–260
Molotoks A, Smith P, Dawson TP (2021) Impacts of land use, population, and climate change on global food security. Food Energy Secur 10:e261
Romano-Armada N, Yanez-Yazlle MF, Irazusta VP, Rajal VB, Moraga NB (2020) Potential of bioremediation and PGP traits in Streptomyces as strategies for bio-reclamation of salt-afected soils for agricultural. Pathogens 9:117
Peng Z, He S, Sun J, Pan Z, Gong W, Lu Y, Du X (2016) Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci Rep 4(6):34548. https://doi.org/10.1038/srep34548
Tanveer M, Ahmed HAI (2020) ROS signaling in modulating salinity stress tolerance in plants. In: Hasanuzzaman M, Tanveer M (eds) Salt and drought stress tolerance in plants. Springer, Cham, pp 299–314
Noureen Z, Zulfiqar AR, Saqib M (2020) Effect of salinity stress on various growt and physioloical attributes of two contrasting Maize genotypes. Agri Agribusi Biotech 63:e20200072
Tounsi S, Feki K, Hmidi D, Masmoudi K, Brini F (2017) Salt stress reveals differential physiological, biochemical and molecular responses in T. monococcum and T. durum wheat genotypes. Physiol Mol Biol Plants 23(3):517–528
Kumar RR, Sharma SK, Goswami S, Singh K, Gadpayle KA, Singh GP, Pathak H, Rai RD (2013) Transcript profiling and biochemical characterization of mitochondrial superoxide dismutase (mtSOD) in wheat (Triticum aestivum) under different exogenous stresses. Aust J Crop Sci 7:414–424
Zelm EV, Zhang Y, Testerink C (2020) Salt tolerance mechanism in plants. Ann Rev Plant Biol 71:403–433
Tokihiko N, Miki F, Motoaki S, Tomohiko K, Satoshi T, Kazuo S (2003) Toxicity of free proline revealed in an Arabidiopsis T-DNA tagged mutant deficient in proline deydroenase. Plant Cell Physiol 44(5):541–548
Hernandez JA, Del Rio LA, Sevilla F (1994) Salt stress induced changes in superoxide dismutase isozyme in leaves and mesophyll protoplast from Vigna unguiculata (L) Walp. New Phytol 126:37–44
Hernandez JA, Olmos E, Corpas FJ, Sevilla F, Del Rio LA (1995) Salt induced oxidative stress in chloroplast of pea plants. Plant Sci 105:151–167
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Mandhania S, Madan S, Sawhney V (2006) Antioxidant defense mechanism under salt stress in wheat seedling. Biol Plant 227:227–231
Ruiz JM, Blasco B, Rivero RM, Romero L (2005) Nicotine‐free and salt‐tolerant tobacco plants obtained by grafting to salinity‐resistant rootstocks of tomato. Physiol Plant 124(4):465–475.
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
Slesak I, Libik M, Karpinska B, Karpinski S, Miszalski Z (2007) The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim Pol 5:39–50
Ahmad P, Jaleel CA, Sharma S (2010) Antioxidant defence system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ J Plant Physiol 57:509–517
Karolina D, Magdalena Z, Andreas B, Hubert S, Krzysztof K, Michal N (2019) Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short term osmotic stress. Sci Rep 9:2743
Xu FJ, Jin CW, Liu WJ, Zhang YS, Lin XY (2011) Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J Integr Plant Biol 53:44–53
Caverzan C, Bonifacio A, Carvalho FEL, Andrade CMB, Passaia G, Schünemann M, Maraschin FS, Martins MO, Teixeira FK, Rauber R et al (2014) The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice. Plant Sci 214:74–87
Scandalios JG, Guan L, Polidoros AN (1997) Catalases in plants: gene structure, properties, regulation, and expression. Cold Spring Harb Monogr Ser 34:343–406
Menezes BL, Teixeira FK, Kamei CLA, Margis PM (2004) Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci 166(2):323–331
Azevedo NA, Prisco JT, Filho JE, Lacerda CF, Silva JV, Costa PH, Filho EG (2004) Effects of salt stress on plant growth, stomatal response and solute accumulation of different maize genotypes. Braz J Plant Physiol 16:31–38
Koca H, Bor M, Ozdemir F, Turkan I (2007) Effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 2007(60):344–351
Mehdi R, Mojtaba K, Fereshteh MH, Sanam SC (2021) Antioxidant gene expression analysis and evaluation of total phenol content and oxygen scavenging system in tea accessions under normal and drought stress conditions. BMC Plant Biol 21:494
Doroskov AV, Bobroviskikah AV (2018) Using the method of systems biology for predicting perspective target genes to select C3 and C4 cereals for oxidative stress resistance. Vavilovskii Zhurnal Genetiki I Selekitsii 22(1):122–131
Raza A, Sharif Y, Chen K, Wang L, Fu H, Zhuang Y, Zhuang W (2022) Genome-wide characterization of ascorbate peroxidase (APX) gene family in peanut (Arachis hypogea L.) revealed their crucial role in growth and multiple stress tolerance. Front Plant Sci 2466
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35:1011–1019
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:23681
Epstein E, Norlyn JD, Rush DW, Kingburry RW, Kelley DB, Cunnigham GA, Wrona AF (1980) Saline culture of crops: a genetic approach. Science 210:399–404
Liang X, Zhang L, Natarajan SK, Becker DF (2013) Proline mechanism of stress survival. Antioxid Redox Signal 19(9):998–1011
Pitman MG, Lauchli A (2002) Global impact of salinity and agricultural ecosystems. In: Lauchli A, Luttge V (eds) Salinity: environment-plants molecules. Kluwer, Dordrecht, pp 375–378
Rengasamy P (2010) Osmotic and ionic effects of various electrolytes on the growth of wheat. Aust J Soil Res 48:120–124
Sairam RKK, Chandrasekhar V, Srivastava GCC (2001) Comparison of hexaploid and tetraploid wheat cultivars in their responses to water stress. Biol Plant 44:89–94
Acknowledgements
The authors would like to acknowledge the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for their technical support RGP.2/169/42.
Funding
Funding for research was provided from project number RGP.2/169/42, King Saud University, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
MR, Conceptualization and Validation; MG, Performed experiments; AAS, Statistical analysis, review and drafting; RTA, Review; MJ, Writing; SU, Drafting and funding support.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramzan, M., Gillani, M., Shah, A.A. et al. Triticum aestivum: antioxidant gene profiling and morpho-physiological studies under salt stress. Mol Biol Rep 50, 2569–2580 (2023). https://doi.org/10.1007/s11033-022-07990-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07990-1