Abstract
Background
Neurological disorders are structural, biochemical, and electrical abnormalities that affect the peripheral and central nervous systems. Paralysis, muscle weakness, tremors, spasms, and partial or complete loss of sensation are some symptoms of these disorders. Neurorehabilitation is the main treatment for neurological disorders. Treatments can improve the quality of life of patients. Neuroprotective substances of natural origin are used for the treatments of these disorders.
Methods and Results
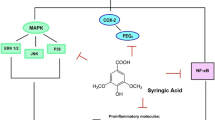
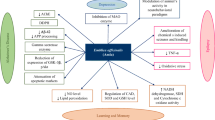
Online databases, such as Google Scholar, PubMed, ScienceDirect, and Scopus were searched to evaluate articles from 1981-2021 using the Mesh words of geraniol (GER), neurological disorders, epilepsy, spinal cord injury (SCI), Parkinson’s diseases (PD), and depression. A total of 87 studies were included in this review. GER with antioxidant, anti-inflammatory, and neuroprotective effects can improve the symptoms and reduce the progression of neurological diseases. GER exhibits neuroprotective effects by binding to GABA and glycine receptors as well as by inhibiting the activation of nuclear factor kappa B (NF-κB) pathway and regulating the expression of nucleotide-binding oligomerization of NLRP3 inflammasome. In this study, the effect of GER was investigated on neurological disorders, such as epilepsy, SCI, PD, and depression.
Conclusion
Although the medicinal uses of GER have been reported, more clinical and experimental studies are needed to investigate the effect of using traditional medicine on improving lifethreatening diseases and the quality of life of patients.





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- WHO:
-
World Health Organization
- PD:
-
Parkinson’s disease
- SCI:
-
Spinal cord injury
- GABAA:
-
Gamma-aminobutyric acid receptor type A
- EO:
-
Essential oil
- PTZ:
-
Pentylenetetrazole
- PIC:
-
Picrotoxin
- GABA:
-
Gamma-aminobutyric acid
- STR:
-
Strychnine
- E1:
-
Hydroalcoholic extract
- CIT:
-
Citral
- GER:
-
Geraniol
- NMDAR1:
-
N-methyl-D-aspartate receptor 1
- iNOS:
-
Inducible nitric oxide synthase
- NF-κB:
-
Kappa-light-chain-enhancer of activated B cells
- MAPK:
-
P38 Mitogen-activated protein kinases
- SN:
-
Substantia nigra
- ROS:
-
Reactive oxygen species
- ATP:
-
Adenosine triphosphate
- Cyc 1:
-
Cytochrome c
- α-Syn:
-
α-Synuclein
- MPTP:
-
1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine
- MPP+:
-
1-Methyl-4-phenylpiridinium
- DAT:
-
DA-ergic neurons with dopamine transporter
- CSF:
-
Cerebrospinal fluid
- BBB:
-
Blood–brain barrier
- IL-6:
-
Interleukin
- TNF-α:
-
Tumour necrosis factor
- ASC:
-
Apoptosis-associated specklike protein
- CUMS:
-
Chronic unpredictable mild stress
- CNS:
-
Central nervous system
- HB:
-
Hole board
- OF:
-
Open field
- BIST:
-
Barbiturate-induced sleeping time
- CP:
-
Cisplatin
- MWM:
-
Morris water maze
- TQ:
-
Thymoquinone
- FST:
-
Forced swimming tests
- TST:
-
Tail suspension tests
- NLRP3:
-
Domain-like receptor family pyrin domain-containing 3
References
Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T et al (2016) Global priorities for addressing the burden of mental, neurological, and substance use disorders. World Bank, Washington
Zis P, Hadjivassiliou M (2019) Treatment of neurological manifestations of gluten sensitivity and coeliac disease. Curr Treat Options Neurol 21:1–10
Arumugam A, Thiyagarajan D (2022) Role of nutrition in pathogenesis of neurological disorders. Role of nutrients in neurological disorders. Springer, Singapore, pp 143–58
Organization WH (2006) Neurological disorders: public health challenges. World Health Organization.
Boice JD Jr, Quinn B, Al-Nabulsi I, Ansari A, Blake PK, Blattnig SR et al (2022) A million persons, a million dreams: a vision for a national center of radiation epidemiology and biology. Int J Radiat Biol 98:795–821
Sanders T, Liu Y, Buchner V, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24:15–46
Butler C, Zeman A (2005) Neurological syndromes which can be mistaken for psychiatric conditions. J Neurol Neurosurg Psychiatry 76:i31–i38
Rekha KR, Selvakumar GP, Santha K, Sivakamasundari RI (2013) Geraniol attenuates α-synuclein expression and neuromuscular impairment through increase dopamine content in MPTP intoxicated mice by dose dependent manner. Biochem Biophys Res Commun 440:664–670
Eggersdorfer M (2000) Terpenes. Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim
Danka R, Williams J, Rinderer T (1990) A bait station for survey and detection of honey bees. Apidologie 21:287–292
Pena GA, da Costa Lopes AS, de Morais SHS, do Nascimento LD, Dos Santos FRR, da Costa KS et al (2022) Host-guest inclusion complexes of natural products and nanosystems: applications in the development of repellents. Molecules 27:2519
Hagberg L, Price RW, Zetterberg H, Fuchs D, Gisslén M (2020) Herpes zoster in HIV-1 infection: the role of CSF pleocytosis in secondary CSF escape and discordance. PLoS ONE 15:e0236162
Stork G, Grieco P, Gregson M (1988) Allylic chlorides from allylic alcohols-geranyl chloride. Org Synth 50:638–640
Calzada JG, Hooz J (1988) Geranyl chloride. Org Synth 50:634–637
Takaya H, Ohta T, Si Inoue, Tokunaga M, Kitamura M, Noyori R (2003) Asymmetric hydrogenation of allylic alcohols using BINAP-ruthenium complexes: (S)-(−)-citronellol: 6-octen-1-ol, 3, 7-dimethyl, (S)-. Organic syntheses. John Wiley & Sons Inc, Hoboken, pp 74–74
Piancatelli G, Leonelli F (2003) Oxidation of nerol to neral with iodosobenzene and TEMPO [(Z)-3, 7-Dimethyl-2, 6-octadienal]. Org Synth 83:18–23
Hinger S (2010) Finding the fundamental: shaping identity in gender and sexual orientation based asylum claims. Colum J Gender L 19:367
Shin HW, Jewells V, Hadar E, Fisher T, Hinn A (2014) Review of epilepsy-etiology, diagnostic evaluation and treatment. Int J Neurorehabilit 1:2376–0281
Martinc B, Grabnar I, Vovk T (2014) Antioxidants as a preventive treatment for epileptic process: a review of the current status. Curr Neuropharmacol 12:527–550
Carlini E (2003) Plants and the central nervous system. Pharmacol Biochem Behav 75:501–512
Almeida R, Navarro D, Barbosa-Filho J (2001) Plants with central analgesic activity. Phytomedicine 8:310–322
Nóbrega RDA, Motta SC, Leite JR (2003) Óleos essenciais com propriedades anticonvulsivantes. Bol Latinoam y del Caribe de Plantas Med y Aromát 2:3–6
de Almeida RN, Motta SC, de Brito FC, Catallani B, Leite JR (2004) Anxiolytic-like effects of rose oil inhalation on the elevated plus-maze test in rats. Pharmacol Biochem Behav 77:361–364
Kamal M, Naz M, Jawaid T, Arif M (2019) Natural products and their active principles used in the treatment of neurodegenerative diseases: a review. Orient Pharm Exp Med 19:343–365
Szwajgier D, Borowiec K, Pustelniak K (2017) The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients 9:477
Almeida R (1999) Metodologia para avaliação de plantas com atividade no sistema nervoso central e alguns dados experimentais. Rev Bras Farm 80:72–76
Quintans-Júnior LJ, Souza T, Leite B, Lessa N, Bonjardim L, Santos M et al (2008) Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine 15:619–624
Meldrum B (1981) GABA-agonists as anti-epileptic agents. Adv Biochem Psychopharmacol 26:207–217
Gale K (1992) GABA and epilepsy: basic concepts from preclinical research. Epilepsia 33:S3-12
Game C, Lodge D (1975) The pharmacology of the inhibition of dorsal horn neurones by impulses in myelinated cutaneous afferents in the cat. Exp Brain Res 23:75–84
McGaraughty S, Henry JL (1998) The effects of strychnine, bicuculline, and ketamine onimmersion-inhibited’dorsal horn convergent neurons in intact and spinalized rats. Brain Res 784:63–70
Blanco M, Costa C, Freire A, Santos J Jr, Costa M (2009) Neurobehavioral effect of essential oil of Cymbopogon citratus in mice. Phytomedicine 16:265–270
Silva MR, Ximenes RM, da Costa JGM, Leal LKA, de Lopes AA, de Barros Viana GS (2010) Comparative anticonvulsant activities of the essential oils (EOs) from Cymbopogon winterianus Jowitt and Cymbopogon citratus (DC) Stapf in mice. Naunyn-Schmiedeberg’s Arch Pharmacol 381:415–26
Dhir A (2020) Natural polyphenols in preclinical models of epilepsy. Phytother Res 34:1268–1281
Lins LCR, Santos IMA, dos Passos Menezes P, Saraújo AA, de Souza Nunes R, dos Santos MRV et al (2014) The anticonvulsant effect of geraniol and inclusion complex geraniol: B-cyclodextrin. Bol Latinoam y del Caribe de Plantas Med y Aromát 13:557–65
Melo MS, Santana MTd, Guimarães AG, Siqueira RS, Sousa DPD, Santos R et al (2011) Bioassay-guided evaluation of central nervous system effects of citronellal in rodents. Rev Bras de Farmacogn 21:697–703
Xu L, Liu M-Z, Yang Y-Y, Wang Y, Hua X-X, Du L-X et al (2022) Geraniol enhances inhibitory inputs to the paraventricular thalamic nucleus and induces sedation in mice. Phytomedicine 98:153965
Lee B, Cripps R, Fitzharris M, Wing P (2012) The global map for traumatic spinal cord injury epidemiology: expanding the online global repository: update 2011. Spinal Cord 52(2):110–116
Majdan M, Brazinova A, Mauritz W (2016) Epidemiology of traumatic spinal cord injuries in Austria 2002–2012. Eur Spine J 25:62–73
Baptiste DC, Fehlings MG (2006) Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 23:318–334
Hagg T, Oudega M (2006) Neural degeneration and regeneration myelin and scar-derived inhibition. J Neurotrauma 23:264–280
Kuo C-Y, Liou T-H, Chang K-H, Chi W-C, Escorpizo R, Yen C-F et al (2015) Functioning and disability analysis of patients with traumatic brain injury and spinal cord injury by using the world health organization disability assessment schedule 2.0. Int J Environ Res Public Health 12:4116–27
Lin B, Xu Y, Zhang B, He Y, Yan Y, He MC (2014) MEK inhibition reduces glial scar formation and promotes the recovery of sensorimotor function in rats following spinal cord injury. Exp Ther Med 7:66–72
Turbes C (1997) Repair, reconstruction, regeneration and rehabilitation strategies to spinal cord injury. Biomed Sci Instrum 34:351–356
Reichenfelser W, Hackl H, Hufgard J, Kastner J, Gstaltner K, Gföhler M (2012) Monitoring of spasticity and functional ability in individuals with incomplete spinal cord injury with a functional electrical stimulation cycling system. J Rehabil Med 44:444–449
Vuckovic A, Hasan MA, Fraser M, Conway BA, Nasseroleslami B, Allan DB (2014) Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J Pain 15:645–655
Tederko P, Krasuski M, Ptyushkin P, Selb M, Pawlak K, Skrzypczyk R et al (2013) Need for a comprehensive epidemiologic study of spinal cord injury in Poland: findings from a systematic review. Spinal cord 51:802–808
Lv Y, Zhang L, Li N, Mai N, Zhang Y, Pan S (2017) Geraniol promotes functional recovery and attenuates neuropathic pain in rats with spinal cord injury. Can J Physiol Pharmacol 95:1389–1395
Wang J, Su B, Zhu H, Chen C, Zhao G (2016) Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-κB and p38 MAPK. Exp Ther Med 12:3607–3613
Sartorius N (2001) The economic and social burden of depression. J Clin Psychiatry 62:8–11
Murray CJ, Lopez AD (1997) Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 349:1498–1504
Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A et al (2006) Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63:1209–1216
Maes M (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry 35:664–675
Kennedy SH, Andersen HF, Thase ME (2009) Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin 25:161–175
Schroder K, Zhou R, Tschopp J (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300
Lu M, Yang J-Z, Geng F, Ding J-H, Hu G (2014) Iptakalim confers an antidepressant effect in a chronic mild stress model of depression through regulating neuro-inflammation and neurogenesis. Int J Neuropsychopharmacol 17:1501–1510
Sahin C, Aricioglu F (2013) A novel aspect for depression and cytokine hypothesis:\ʹNLRP3 Inflammasome\ʹ. Clin Exp Health Sci 3:65
Deng X-Y, Xue J-S, Li H-Y, Ma Z-Q, Fu Q, Qu R et al (2015) Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol Behav 152:264–271
Medeiros KAA, Dos Santos JR, Melo TCdS, de Souza MF, Santos LdG, de Gois AM et al (2018) Depressant effect of geraniol on the central nervous system of rats: behavior and ECoG power spectra. Biomed J 41:298–305
Kandeil MA, Gomaa SB, Mahmoud MO (2020) The effect of some natural antioxidants against cisplatin-induced neurotoxicity in rats: behavioral testing. Heliyon 6:e04708
James P (1817) An essay on the shaking palsy. Sherwood Neely and Jones, London
Gelders G, Baekelandt V, Van der Perren A (2018) Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J Immunol Res 2018:1–12
Kempuraj D, Thangavel R, Natteru P, Selvakumar G, Saeed D, Zahoor H et al (2016) Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 1:1003
Büeler H (2009) Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp Neurol 218:235–246
Zuo L, Motherwell MS (2013) The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 532:18–23
Moon HE, Paek SH (2015) Mitochondrial dysfunction in Parkinson’s disease. Exp neurobiol 24:103
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Hartmann A, Hunot S, Michel PP, Muriel M-P, Vyas S, Faucheux BA et al (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA 97:2875–2880
Tatton WG, Chalmers-Redman R, Brown D, Tatton N (2003) Apoptosis in Parkinson’s disease: signals for neuronal degradation. Annals Neurol 53:S61–S72
Hartmann A, Michel PP, Troadec JD, Mouatt-Prigent A, Faucheux BA, Ruberg M et al (2001) Is bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson’s disease? J Neurochem 76:1785–1793
Xiong N, Jia M, Chen C, Xiong J, Zhang Z, Huang J et al (2011) Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neuroscience 199:292–302
Dodson M, Darley-Usmar V, Zhang J (2013) Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med 63:207–221
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441:523–540
Zhang J (2013) Autophagy and mitophagy in cellular damage control. Redox Biol 1:19–23
Braak H, Del Tredici K (2008) Invited article: nervous system pathology in sporadic Parkinson disease. Neurology 70:1916–1925
Zhou J, Broe M, Huang Y, Anderson JP, Gai W-P, Milward EA et al (2011) Changes in the solubility and phosphorylation of α-synuclein over the course of Parkinson’s disease. Acta Neuropathol 121:695–704
Yokoyama H, Kuroiwa H, Yano R, Araki T (2008) Targeting reactive oxygen species, reactive nitrogen species and inflammation in MPTP neurotoxicity and Parkinson’s disease. Neurol Sci 29:293–301
Brooks W, Jarvis M, Wagner G (1989) Astrocytes as a primary locus for the conversion MPTP into MPP+. J Neural Transm 76:1–12
Smeyne RJ, Jackson-Lewis V (2005) The MPTP model of Parkinson’s disease. Mol Brain Res 134:57–66
Olanow C, Tatton W (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123–144
Przedborski S, Jackson-Lewis V (1998) Mechanisms of MPTP toxicity. Move disord 13:35–38
Przedborski S, Chen Q, Vila M, Giasson BI, Djaldatti R, Vukosavic S et al (2001) Oxidative post-translational modifications of α-synuclein in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Neurochem 76:637–640
Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P (2010) Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimer’s Dis 20:S453–S473
Lim K-L, Ng C-H (2009) Genetic models of Parkinson disease. Biochim et Biophys Acta (BBA)-Mol Basis of Dis 1792:604–15
Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too? Curr Biol 27:R713–R715
Wang X, Ye X-l, Liu R, Chen H-L, Bai H, Liang X et al (2010) Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chemico-Biol Interact 184:328–37
Rekha KR, Selvakumar GP, Sethupathy S, Santha K, Sivakamasundari RI (2013) Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson’s disease. J Mol Neurosci 51:851–862
Luo M, Liu X, Zu Y, Fu Y, Zhang S, Yao L et al (2010) Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chemico-Biol Interact 188:151–60
De Fazio L, Spisni E, Cavazza E, Strillacci A, Candela M, Centanni M et al (2016) Dietary geraniol by oral or enema administration strongly reduces dysbiosis and systemic inflammation in dextran sulfate sodium-treated mice. Front Pharmacol 7:38
ChO M, So I, Chun JN, Jeon J-H (2016) The antitumor effects of geraniol: modulation of cancer hallmark pathways. Int J Oncol 48:1772–1782
Pavan B, Dalpiaz A, Marani L, Beggiato S, Ferraro L, Canistro D et al (2018) Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front Pharmacol 9:18
de Oliveira Junior ER, Truzzi E, Ferraro L, Fogagnolo M, Pavan B, Beggiato S et al (2020) Nasal administration of nanoencapsulated geraniol/ursodeoxycholic acid conjugate: towards a new approach for the management of Parkinson’s disease. J Control Release 321:540–552
Rekha KR, Sivakamasundari RI (2018) Geraniol protects against the protein and oxidative stress induced by rotenone in an in vitro model of Parkinson’s disease. Neurochem Res 43:1947–1962
Acknowledgements
The authors are grateful to the staff of the Neurophysiology Research Center, Hamadan University of Medical Sciences for supporting this study.
Funding
The current study was funded (IR.UMSHA.REC.1400.771) by Hamadan University of Medical Sciences, Hamadan, Iran.
Author information
Authors and Affiliations
Contributions
SB, IS, FRA, MKA and AK reviewed the literature and drafted the manuscript. SB and AK critically revised the manuscript. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All authors agree to publish the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bagheri, S., Salehi, I., Ramezani-Aliakbari, F. et al. Neuroprotective effect of geraniol on neurological disorders: a review article. Mol Biol Rep 49, 10865–10874 (2022). https://doi.org/10.1007/s11033-022-07755-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07755-w