Abstract
Background
This study served as the pioneer in studying the anti-cancer role of chicken cathelicidin peptides.
Methods and Results
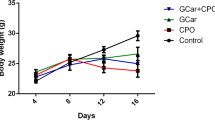
Chicken cathelicidins were used as anticancer agent against the breast cancer cell line (MCF-7) and human colon cancer cell line (HCT116). In addition, the mechanism of action of the interaction of cationic peptides with breast cancer cell line MCF-7 was also investigated. An in vivo investigation was also achieved to evaluate the role of chicken cathelicidin in Ehrlich ascites cell (EAC) suppression as a tumor model after subcutaneous implantation in mice. It was found during the study that exposure of cell lines to 40 µg/ml of chicken cathelicidin for 72 h reduced cell lines growth rate by 90–95%. These peptides demonstrated down-regulation of (cyclin A1 and cyclin D genes) of MCF-7 cells. The study showed that two- and three-fold expression of both of caspase-3 and − 7 genes in untreated MCF-7 cells compared to treated MCF-7 cells with chicken cathelicidin peptides. Our data showed that chicken (CATH-1) enhance releasing of TNFα, INF-γ and upregulation of granzyme K in treated mice groups, in parallel, the tumor size and volume was reduced in the treated EAC-bearing groups. Tumor of mice groups treated with chicken cathelicidin displayed high area of necrosis compared to untreated EAC-bearing mice. Based on histological analysis and immunohistochemical staining revealed that the tumor section in Ehrlich solid tumor exhibited a strong Bcl2 expression in untreated control compared to mice treated with 10 & 20 µg of cathelicidin. Interestingly, low expression of Bcl2 were observed in mice taken 40 µg/mL of CATH-1.
Conclusions
This study drive intention in treatment of cancer through the efficacy of anticancer efficacy of chicken cathelicidin peptides.








Similar content being viewed by others
Data Availability Statement
All data used in this study were granted in United States Patent and Trademark office under patent number (US 11179438B1).
References
Kuroda K, Fukuda T, Isogai H, Okumura K, Krstic-Demonacos M, Isogai E (2015) Antimicrobial peptide FF/CAP18 induces apoptotic cell death in HCT116 colon cancer cells via changes in the metabolic profile. Int J Oncol 46:1516–1526. doi:https://doi.org/10.3892/ijo.2015.2887
Parvy J-P, Yu Y, Dostalova A, Kondo S, Kurjan A, Bulet P, Lemaître B, Vidal M, Cordero JB (2019) The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila. eLife 8, e45061
Wu WK, Wang G, Coffelt SB, Betancourt AM, Lee CW, Fan D et al (2010) Emerging roles of the host defense peptide LL-37 in human cancer and its potential therapeutic applications. Int J Cancer 127(8):1741–1747. doi:https://doi.org/10.1002/ijc.25489
Wang G, Mishra B, Epand RF, Epand RM (2014) High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim Biophys Acta 1838(9):2160–2172
Goitsuka R, Chen C-LH, Benyon L, Asano Y, Kitamura D, Cooper MD (2007) Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc. Natl. Acad. Sci. USA 104, 15063–15068
Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, Zhang G (2006) Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem 281:2858–2867
Achanta M, Sunkara LT, Dai G, Bommineni YR, Jiang W, Zhang (2012) G. Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides. J Anim Sci Biotechnol 3:15
Cuperus T, Van Dijk A, Matthijs MGR, Veldhuizen EJA, Haagsman HP (2016) Protective effect of in ovo treatment with the chicken cathelicidin analog D-CATH-2 against avian pathogenic E. coli. Sci. Rep. 6, 26622
Cuperus T, Coorens M, Van Dijk A, Haagsman HP (2013) Avian host defense peptides. Dev Comp Immunol 41:352–369
Lee MO, Jang H-J, Rengaraj D, Yang S-Y, Han JY, Lamont SJ, Womack JE (2016) Tissue expression and antibacterial activity of host defense peptides in chicken. BMC Vet Res 12:231
Lee MO, Kim E-H, Jang H-J, Na Park M, Woo H-J, Han JY, Womack JE (2012) Effects of a single nucleotide polymorphism in the chicken NK-lysin gene on antimicrobial activity and cytotoxicity of cancer cells. Proc. Natl. Acad. Sci. USA 109, 12087–12092
Zasloff M (2019) Antimicrobial Peptides of Multicellular Organisms: My Perspective. Adv Exp Med Biol 1117:3–6
Van Dijk A, Tersteeg-Zijderveld MH, Tjeerdsma-van Bokhoven JL, Jansman AJ, Veldhuizen EJ, Haagsman HP (2009) Chicken heterophils are recruited to the site of Salmonella infection and release antibacterial mature Cathelicidin-2 upon stimulation with. LPS Mol Immunol 46:1517–1526
Van Dijk A, van Eldik M, Veldhuizen EJ, Tjeerdsma-van Bokhoven HL, de Zoete MR, Bikker FJ, Haagsman HP (2016) Immunomodulatory and anti-inflammatory activities of chicken cathelicidin-2 derived peptides. PLoS ONE 11:e0147919
Coorens M, Van Dijk A, Bikker F, Veldhuizen EJA, Haagsman HP (2015). Importance of endosomal cathelicidin degradation to enhance DNA-induced chicken macrophage activation. J Immunol 195:3970–3977
Coorens M, Schneider VA, de Groot AM, van Dijk A, Meijerink M, Wells JM, Scheenstra MR, Veldhuizen EJ, Haagsman HP 2017 Cathelicidins inhibit escherichia coli–induced tlr2 and tlr4 activation in a viability-dependent manner.J Immunol199(4):1418–1428
Peng L, Du W, Balhuizen M, Haagsman HP, de Haan C, Veldhuizen (2020) E.J.A. Antiviral activity of chicken cathelicidin B1 against influenza A virus. Front Microbiol 11:426
Peng L, Scheenstra MR, van Harten RM, Haagsman HP, Veldhuizen EJ (2020) The immunomodulatory effect of cathelicidin-B1 on chicken macrophages. Vet Res 51:122
Chen X, Zou X, Qi G, Tang Y, Guo Y, Si J, Liang L (2018) Roles and Mechanisms of Human Cathelicidin LL-37 in Cancer. Cell Physiol Biochem 47:1060–1073
Blagosklonny MV, Pardee AB (2001) Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res 61:4301–4305
Nurse P (2002) Cyclin dependent kinases and cell cycle control (Nobel lecture). ChemBioChem 3, 596–603
Ghosh R, Ott AM, Seetharam D, Slaga TJ, Kumar AP (2003) Cell cycle block and apoptosis induction in a human melanoma cell line following treatment with 2-methoxyoestradiol:. Therapeutic implications? Melanoma Res 13:119–127
Johansson J, Gudmundsson GH, Rottenberg M, Berndt K, Agerberth B (1998) Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem 273:3718–3724. doi:https://doi.org/10.1074/jbc.273.6.3718
Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y (1999) Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: Relevance to the molecular basis for its non-cell-selective activity. Biochem J 341(Pt 3):501–513. doi:https://doi.org/10.1042/0264-6021:3410501
Dennison SR, Whittaker M, Harris F, Phoenix DA (2006) Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr Protein Pept Sci 7:487–499. doi:https://doi.org/10.2174/138920306779025611
Yacoub HA, Elazzazy A, Mahmoud MM, Baeshen MN, Al-Maghrabi OA, Alkarim S, Ahmed ES, Almehdar HA, Uversky VN (2016). Chicken cathelicidins as potent intrinsically disordered biocides with antimicrobial activity against infectious pathogens. Dev Comp Immunol 65:8–24
Chen Y, Mant CT, Farmer SW, Hancock R, Vasil ML, Hodges RS (2005) Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem 280:12316–12329. doi:https://doi.org/10.1074/jbc.M413406200
Huang Y, Wang X-F, Wang H-Y, Liu Y, Chen Y (2011) Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol Cancer Ther 10:416–426. doi:https://doi.org/10.1158/1535-7163. MCT-10-0811
Isogai E, Isogai H, Matuo K, Hirose K, Kowashi Y, Okumuara K et al (2003) Sensitivity of genera Porphyromonas and Prevotella to the bactericidal action of C-terminal domain of human CAP18 and its analogues. Oral Microbiol Immunol 18(5):329–332. doi:https://doi.org/10.1034/j.1399-302X.2003.00083.x
Riedl S, Zweytick D, Lohner (2011) K. Membrane-active host defense peptides—Challenges and perspectives for the development of novel anticancer drugs. Chem Phys Lipids 164:766–781. doi:https://doi.org/10.1016/j.chemphyslip.2011.09.004
Harris F, Dennison SR, Singh J, Phoenix DA On the selectivity and efficacy of defense peptides with respect to cancer cells.Med Res Rev2013, 33(1):190–234. doi: https://doi.org/10.1002/med.20252
Simons K, Ikonen E (2000) How cells handle cholesterol. Science 290, 1721–1726, doi:https://doi.org/10.1126/science.290.5497.1721
Matsuzaki K, Sugishita K, Fujii N, Miyajima K (1995) Molecular basis for membrane selectivity of an antimicrobial peptide, magainin 2. Biochemistry 34, 3423–3429, doi:https://doi.org/10.1021/bi00010a034
Wojcik C, Sawicki W, Marianowski P, Benchaib M, Czyba JC, Guerin JF Cyclodextrin enhances spermicidal effects of magainin-2-amide. Contraception 2000, 62, 99–103, doi:https://doi.org/10.1016/S0010-7824(00)00143-8
Steiner H, Andreu D, Merrifield RB (1988) Binding and action of cecropin and cecropin analogues: Antibacterial peptides from insects. Biochim Biophys Acta 939:260–266. doi:https://doi.org/10.1016/0005-2736(88)90069-7
Li Y, Li X, Wang G (2006) Cloning, expression, isotope labeling, and purification of human antimicrobial peptide LL-37 in Escherichia coli for NMR studies. Protein Expr Purif 47:498–505
Higgins GS, O’Cathail SM, Muschel R, McKenna WG (2015) Drug radiotherapy combinations: Review of previous failures and reasons for future optimism. Cancer Treat Rev 41:105–113. doi:https://doi.org/10.1016/j.ctrv.2014.12.012
Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G (2010) Recent advances in cancer therapy: An overview. Curr Pharm Des 16:3–10. doi:https://doi.org/10.2174/138161210789941847
Ciruelos Gil EM (2014) Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev 40(7):862–871. 10.1016/j. ctrv.2014.03.004
Vincenzi B, Imperatori M, Silletta M, Marrucci E, Santini D, Tonini G (2015) Emerging kinase inhibitors of the treatment of gastric cancer. Expert Opin Emerg Drugs 20:479–493. doi:https://doi.org/10.1517/14728214.2015.1051467
Karczmarek-Borowska B, Sałek-Zań A (2015) Hepatotoxicity of molecular targeted therapy. Contemp Oncol 19:87–92. https://doi.org/10.5114/wo.2014.4349544
Ray G, Dhar G, Van Veldhuizen PJ, Banerjee S, Saxena NK, Sengupta K, Banerjee SK (2006) Modulation of cell-cycle regulatory signaling network by 2-. pathways Biochem 45:3703–3713methoxyestradiol in prostate cancer cells is mediated through multiple signal transduction
Pathania D, Millard M, Neamati N (2009) Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev 61:1250–1275. doi:https://doi.org/10.1016/j.addr.2009.05.0105
Li Y, Cozzi PJ (2010) Angiogenesis as a strategic target for prostate cancer therapy. Med Res Rev 30:23–66. doi:https://doi.org/10.1002/med.20161
Breen EC, Walsh J (2010) Tubulin-targeting agents in hybrid drugs. Curr Med Chem 17:609–639. doi:https://doi.org/10.2174/092986710790416254
Udenigwe CC, Aluko RE (2012) Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77(1):R11–24. https://doi.org/10.1111/j.1750-3841.2011.02455.x54
Zheng LH, Wang YJ, Sheng J, Wang F, Zheng Y, Lin XK et al Antitumor peptides from marine organisms.Mar Drugs2011, 9(10):1840–59. doi: https://doi.org/10.3390/md910184055
Smolarczyk R, Cichon T, Szala S (2009) [Peptides: a new class of anticancer drugs]. Postepy Hig Med Dosw (Online) 63:360–368
Liu W, Min Hu M, Wang Y, Sun B, Guo Y, Xu Z, Li J, Han B Overexpression of interleukin-18 protein reduces viability and induces apoptosis of tongue squamous cell carcinoma cells by activation of glycogen synthase kinase-3β signaling 2015, 33(3):1049–56. doi: https://doi.org/10.3892/or.2015.3724
Liu W, Han B, Sun B, Gao Y, Huang Y, Hu M (2012) Overexpression of interleukin-18 induces growth inhibition, apoptosis and gene expression changes in a human tongue squamous cell carcinoma cell line. J Int Med Res 40:537–544
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408
Lee MO, Jang HJ, Rengaraj D, Yang SY, Han JY, Lamont SJ, Womack JE (2016) Tissue expression and antibacterial activity of host defense peptides in chicken. BMC Vet Res 12:231
Frajacomo FTT, Padilha C, Marinello PC, Guarnier F, Cecchini R, Duarte JA, Deminice R (2016) Solid Ehrlich carcinoma reproduces functional and biological characteristics of cancer cachexia. Life Sci 162:47–53
Mader JS, Mookherjee N, Hancock R, Bleackley RC (2009) The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factordependent manner involving Bax activity. Mol Cancer Res 7:689–702. doi:https://doi.org/10.1158/1541-7786.MCR-08-0274
Stander BA, Marais S, Vorster C, Joubert (2010), A.M. In vitro effects of 2-methoxyestradiol on morphology, cell cycle progression, cell death and gene expression changes in the tumorigenic MCF-7 breast epithelial cell line. J Steroid Biochem Mol Biol 119:149–160
Choi HJ, Zhu BT Critical role of cyclin B1/Cdc2 up-regulation in the induction of mitotic prometaphase arrest in human breast cancer cells treated with 2-methoxyestradiol.Biochim Biophys Acta2012, 1823(8):1306-15.doi: https://doi.org/10.1016/j.bbamcr.2012.05.003
Hartwell LH, Weinert TA, Checkpoints (1989) Controls that Ensure the Order of Cell Cycle Events. Science 246:629–634
Van Azevedo PV, Lopes F, Zóia DS, Correia MAP, Saito LIV, Fonseca N, Polloni BB, Teixeira L, Goulart SC (2022) Melo Rodrigues Ávila, V. A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules 12:258. https://doi.org/10.3390/biom12020258. de
Wesierska-Gadek J, Gueorguieva M, Wojciechowski J, Horky M (2004) Cell cycle arrest induced in human breast cancer cells by cyclin-dependent kinase inhibitors: a comparison of the effects exerted by roscovitine and olomoucine. Pol J Pharmacol 56(5):635–641
Kuroda K, Fukuda T, Yoneyama H, Katayama M, Isogai H, Okumura K, Isogai E (2012) Anti-proliferative effect of an analogue of the LL-37 peptide in the colon cancer derived cell line HCT116 p53+/+ and p53. Oncol Rep 28:829–834. doi:https://doi.org/10.3892/or.2012.1876
Yang Y-H, Zheng G-G, Li G, Zhang B, Song Y-H, Wu K-F (2003) Expression of LL-37/hCAP-18 gene in human leukemia cells. Leuk Res 27:947–950. https://doi.org/10.1016/S0145-2126(03)00020-189
Ren SX, Shen J, Cheng A, Lu L, Chan RLY, Li ZJ, Wang XJ, Wong CCM, Zhang L, Ng SSM et al (2013) FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS ONE 8:e63641. doi:https://doi.org/10.1371/journal.pone.0063641
Savarese DM, Savy G, Vahdat L, Wischmeyer E, Corey P (2003) Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev 29:501–513
Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Joost J, Oppenheim, Chertov O (2000) “LL-37, the neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells”. J Exp Med 192(7):1069–1074
Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G (2002) Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84(2–3):215–222. doi: https://doi.org/10.1016/s0300-9084(02)01374-3
Ni L, Lu JInterferon gamma in cancer immunotherapy.Cancer Medicine2018,7,4509–4516
Chuang CM, Monie A, Wu A, Mao CP, Hung CF (2009) Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther 20(4):303–313. 10.1089/ hum.124
Bucki R, Leszczyńska K (2010) Andrzej Namiot, and Wojciech Sokołowski. “Cathelicidin LL-37: a multitask antimicrobial peptide.“ Archivum immunologiae et therapiae experimentalis58 (1):15–25
Bots M, Medema JP (2006) Granzymes at a glance. J Cell Sci 119:5011–5014
Mader JS, Catherine Ewen REW, Hancock, Robert C, Bleackley “The human cathelicidin, LL-37, induces granzyme-mediated apoptosis in regulatory T cells.“Journal of Immunotherapy2011,34 (3):229–235
Cheng M, Ho S 1, Yoo JH, Tran DH, Bakirtzi K, Su B, Tran DHY, Kubota IchikawaR, Koon HW (2015) Cathelicidin suppresses colon cancer development by inhibition of cancer associated fibroblasts. Clin Exp Gastroenterol 8:13–29
Piktel E, Niemirowicz K, Wnorowska U, Wątek M, Wollny T, Głuszek K, Góźdź S, Levental I, Bucki R (2016) The role of cathelicidin LL-37 in cancer development. Arch Immunol Ther Exp 64:33–46
Wang C, Dong S, Zhang L, Zhao Y, Huang L, Gong X, Wang H, Shang D Cell surface binding, uptaking and anticancer activity of L-K6, a lysine/leucine-rich peptide, on human breast cancer MCF-7 cells. Scientific Reports 2017,7:8293. DOI: https://doi.org/10.1038/s41598-017-08963-2, PMID: 28811617
Acknowledgements
The authors thank King Abdulaziz City for Science and Technology for technical and financial support under grant no. (14-Bio883-03). The authors are solely responsible for the contents of this report. In addition, the authors thanks King Abdulaziz University, Science and technology unit (STU) for technical and financial support and where the research was achieved at its laboratories.
Funding
King Abdulaziz City for Science and Technology.
Author information
Authors and Affiliations
Contributions
H.A.Y. contributed to the study design, performed experiments, analyzed the data, and wrote the manuscript; M.A. and M.M.M. contributed to the data analysis and revised the manuscript; F.A. contributed to cell culture and RT-PCR analysis; A.M.A.-H., T.S.A., M.A. and A.N. provided in vivo experiments; M.A. performed flow cytometry analysis; I.A.A. provided Ultrasounds experiment, H.A.Y. contributed to gene expression studies; H.A.Y designed the study, supervised the experiments, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional Review Board Statement
the Ethics Committee of King Abdulaziz University (Reference No 325 − 19).
Conflict of interest
All the authors declare no conflict of interest. All data used in this study were granted in United States Patent and Trademark office under Patent number (US 11179438B1). The manuscript has submitted as a preprint in Research Square platform under DOI: https://doi.org/10.21203/rs.3.rs-447791/v1.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahmoud, M.M., Alenezi, M., Al-Hejin, A.M. et al. Anticancer activity of chicken cathelicidin peptides against different types of cancer. Mol Biol Rep 49, 4321–4339 (2022). https://doi.org/10.1007/s11033-022-07267-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07267-7