Abstract
Background
The serum and glucocorticoid-induced kinase-1 (SGK1) belonging to the AGC protein kinase family phosphorylates serine and threonine residues of target proteins. It regulates numerous ion channels and transporters and promotes survival under cellular stress. Unique to SGK1 is a tight control at transcriptional and post-transcriptional levels. SGK1 regulates multiple signal transduction pathways related to tumor development. Several studies have reported that SGK1 is upregulated in different types of human malignancies and induces resistance against inhibitors, drugs, and targeted therapies.
Results and Conclusion
This review highlights the cellular functions of SGK1, its crucial role in cancer development, and clinical insights for SGK1 targeted therapies. Furthermore, the role of SGK1-mediated autophagy as a potential therapeutic target for cancer has been discussed.




Similar content being viewed by others
Abbreviations
- SGK:
-
Serum and glucocorticoid-induced kinase-1
- AKT:
-
Protein kinase B
- PKA:
-
CAMP dependent protein kinase
- PDK1:
-
Phosphoinositide dependent protein kinase-1
- PKG:
-
CGMP dependent protein kinase
- S6K:
-
Ribosomal S6 kinase
- PKC:
-
Protein kinase C
- MAST:
-
Microtubule-associated serine/threonine kinase
- ROCK:
-
Rho-associated protein kinase
- YANK:
-
Yet another novel kinase
- GRK:
-
G protein-coupled receptor kinase
- Nt:
-
Amino-terminus
- Ct:
-
Carboxy-terminus
- ER:
-
Endoplasmic reticulum
- PIP3 :
-
Phosphatidylinositol-trisphosphate
- TGFβ:
-
Growth factor beta
- FSH:
-
Follicle stimulating hormone
- ENaC:
-
Epithelial sodium channel
- Th1:
-
Type-1 T helper cells
- Tregs:
-
Regulatory T cells
- ECM:
-
Extra cellular matrix
- TME:
-
Tumor microenvironment
- mTORC1:
-
Mammalian target of rapamycin complex-1
- YAP:
-
Yes-associated protein
- GLI1:
-
GLI family zinc finger-1
- MTA1:
-
Metastasis-associated protein-1
- PIN1:
-
Peptidyl-prolyl cis/trans isomerase NIMA-interacting-1
- TAMs:
-
Tumor-associated macrophages
- APC:
-
Adenomatous polyposis coli
- shRNA:
-
Short hairpin RNA
- CMA:
-
Chaperone-mediated autophagy
- LAMP2A:
-
Lysosomal-associated membrane protein-2A
- LC3B2:
-
Microtubule-associated proteins 1A/1B light chain-3B2
- FOXO3:
-
Forkhead box O3
- ULK1:
-
Unc-51 like autophagy activating kinase-1
- BECN1:
-
Beclin-1
References
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619. https://doi.org/10.1038/nrg1879
Samuels Y, Zhenghe W, Alberto B, Natalie S, Janine P, Steve S, Hai Y, Adi G, Steven MP, Gregory JR (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304:554. https://doi.org/10.1126/science.1096502
Fruman DA, Honyin C, Benjamin DH, Shubha B, Lewis CC, Robert TA (2017) The PI3K pathway in human disease. Cell 170:605–635. https://doi.org/10.1016/j.cell.2017.07.029
Kondapalli L, Soltani K, Lacouture ME (2005) The promise of molecular targeted therapies: protein kinase inhibitors in the treatment of cutaneous malignancies. J Am Acad Dermatol 53:291–302. https://doi.org/10.1016/j.jaad.2005.02.011
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298:1912–1934. https://doi.org/10.1126/science.1075762
Maurer M (2009) 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res 69:6299–6306. https://doi.org/10.1158/0008-5472.CAN-09-0820
Kobayashi T, Deak M, Morrice N, Cohen P (1999) Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 344:189–197
Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL (1993) Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13:2031–2040. https://doi.org/10.1128/mcb.13.4.2031
Waldegger S, Barth P, Raber G, Lang F (1997) Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA 94:4440–4445. https://doi.org/10.1073/pnas.94.9.4440
Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V (2006) (Patho) physiological significance of the serum- and glucocorticoidinducible kinase isoforms. Physiol Rev 86:1151–1178. https://doi.org/10.1152/physrev.00050.2005
Rauz S, Walker EA, Hughes SV, Coca-Prados M, Hewison M, Murray PL, Stewart PM (2003) Serum- and glucocorticoid-regulated kinase isoform-1 and epithelial sodium channel subunits in human ocular ciliary epithelium. IOVS 44:1643–1651. https://doi.org/10.1167/iovs.02-0514
Wang HW, Huang BS, Chen A, Ahmad M, White RA, Leenen FH (2016) Role of brain aldosterone and mineralocorticoid receptors in aldosterone-salt hypertension in rats. Neuroscience 314:90–105. https://doi.org/10.1016/j.neuroscience.2015.11.055
Sahin P, McCaig C, Jeevahan J, Murray JT, Hainsworth AH (2013) The cell survival kinase SGK1 and its targets FOXO3a and NDRG1 in aged human brain. Neuropathol Appl Neurobiol 39:623–633. https://doi.org/10.1111/nan.12023
Lee E, Lein ES, Firestone GL (2001) Tissue-specific expression of the transcriptionally regulated serum and glucocorticoid-inducible protein kinase (Sgk) during mouse embryogenesis. Mech Dev 103:177–181. https://doi.org/10.1016/s0925-4773(01)00351-3
Waldegger S, Klingel K, Barth P, Sauter M, Rfer ML, Kandolf R, Lang F (1999) h-sgk serine-threonine protein kinase gene as transcriptional target of transforming growth factor beta in human intestine. Gastroenterology 116(5):1081–1088. https://doi.org/10.1016/s0016-5085(99)70011-9
Yu XB, Lin Q, Qin X, Ruan Z, Zhou JH, Lin ZF, Su YJ, Jian Z (2016) Serum and glucocorticoid kinase 1 promoted the growth and migration of non-small cell lung cancer cells. Gene 576:339–346. https://doi.org/10.1016/j.gene.2015.10.072
Yaylaoglu MB, Agbemafle BM, Oesterreicher TJ, Finegold MJ, Thaller C, Henning SJ (2006) Diverse patterns of cell-specific gene expression in response to glucocorticoid in the developing small intestine. Am J Physiol Gastrointest Liver Physiol 291:G1041–G1050. https://doi.org/10.1152/ajpgi.00139.2006
Hou JH, Speirs HJ, Seckl JR, Brown RW (2002) Sgk1 gene expression in kidney and its regulation by aldosterone: Spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol 13:1190–1198. https://doi.org/10.1097/01.ASN.0000013702.73570.3B
Klingel K, Warntges S, Bock J, Wagner CA, Sauter M, Waldegger S, Kandolf R, Lang F (2000) Expression of cell volume-regulated kinase h-sgk in pancreatic tissue. Am J Physiol Gastrointest Liver Physiol 279:G998–G1002. https://doi.org/10.1152/ajpgi.2000.279.5.G998
Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Peggie M, Bain J, Bloomberg GB, Grahammer F (2004) Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J 84:477–488. https://doi.org/10.1042/BJ20041057
Chen L, Wei TQ, Wang Y, Zhang J, Li H, Wang KJ (2012) Simulated bladder pressure stimulates human bladder smooth muscle cell proliferation via the PI3K/SGK1 signaling pathway. J Urol 188:661–667. https://doi.org/10.1016/j.juro.2012.03.112
Li P, Pan F, Hao Y, Feng W, Song H, Zhu D (2013) SGK1 is regulated by metabolicrelated factors in 3T3-L1 adipocytes and overexpressed in the adipose tissue of subjects with obesity and diabetes. Diabetes Res Clin Pract 102:35–42. https://doi.org/10.1016/j.diabres.2013.08.009
Sun JY, Li C, Shen ZX, Zhang WC, Ai TJ, Du LJ, Zhang YY, Yao GF, Liu Y, Sun S (2016) Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-B pathways. Arterioscler Thromb Vasc Biol 36:874–885. https://doi.org/10.1161/ATVBAHA.115.307031
Pelzl L, Fakhri H, Umbach AT, Gawaz M, Paulmichl M, Lang F (2013) Sgk1 sensitive pendrin expression in murine platelets. Cell Physiol Biochem 32:210–220. https://doi.org/10.1159/000356640
Arteaga MF, Alvarez R, Alvar JA (2007) Multiple translational isoforms give functional specificity to serum- and glucocorticoid-induced kinase 1. Mol Biol Cell 18:2072–2080. https://doi.org/10.1091/mbc.E06-10-0968
Bogusz MA, Brickley DR, Pew T, Conzen SD (2006) A novel N-terminal hydrophobic motif mediates constitutive degradation of serum and glucocorticoid-induced kinase-1 by the ubiquitin-proteasome pathway. FEBS J 273:2913–2928. https://doi.org/10.1111/j.1742-4658.2006.05304.x
Zhou R, Snyder PM (2005) Nedd4-2 phosphorylation induces serum and glucocorticoid-regulated kinase (SGK) ubiquitination and degradation. JBC 280:4518–4523. https://doi.org/10.1074/jbc.M411053200
Pao AC (2012) SGK regulation of renal sodium transport. Curr Opin Nephrol Hypertens 2:534–540. https://doi.org/10.1097/MNH.0b013e32835571be
Daniela R, Olivier S (2012) Nedd4-2 and the regulation of epithelial sodium transport. Front Physiol 3:212. https://doi.org/10.3389/fphys.2012.00212
Yang N, Jiang J, Deng L, Waters MJ, Wang X, Frank SJ (2010) Growth hormone receptor targeting to lipid rafts requires extracellular subdomain 2. Biochem Biophys Res Commun 391:414–418. https://doi.org/10.1016/j.bbrc.2009.11.072
Diego AR, Ignacio G, Biff F, Cecilia MC (2006) SGK1 activates Na+-K+-ATPase in amphibian renal epithelial cells. AJP Cell Physiol 290(2):C492-498. https://doi.org/10.1152/ajpcell.00556.2004
Du YN, Tang XF, Xu L, Chen WD, Gao PJ, Han WQ (2018) SGK1-FoxO1 signaling pathway mediates Th17/Treg imbalance and target organ inflammation in angiotensin II-induced hypertension. Front Physiol 9:1581. https://doi.org/10.3389/fphys.2018.01581
Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J et al (2002) Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Investig 110:1263–1268. https://doi.org/10.1172/JCI15696
Sarah I, Shannon H, Philip V (2015) Serum- and glucocorticoid-inducible kinase 1 confers protection in cell-based and in in vivo neurotoxin models via the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol 35:1192–2206. https://doi.org/10.1128/MCB.01510-14
Ackermann TF, Boini KM, Beier N, Scholz W, Fuchss T, Lang F (2011) EMD638683, a novel SGK inhibitor with antihypertensive potency. Cell Physiol Biochem 28:137–146. https://doi.org/10.1159/000331722
Nishida Y, Nagata T, Takahashi Y, Sugahara-Kobayashi M, Murata A, Asai S (2004) Alteration of serum/glucocorticoid regulated kinase-1 (sgk-1) gene expression in rat hippocampus after transient global ischemia. Brain Res Mol Brain Res 123(1–2):121–125. https://doi.org/10.1016/j.molbrainres.2004.01.008
Zhuang X, Zhang H, Hu G (2019) Cancer and microenvironment plasticity: double-edged swords in metastasis. Trends Pharmacol Sci 40:419–429. https://doi.org/10.1016/j.tips.2019.04.005
Conza D, Paola M, Gaetano TT, Luigi I, Francesca F, Silvia S, Rosario A, Francesco B, Nicola P et al (2017) The SGK1 inhibitor SI113 induces autophagy, apoptosis, and endoplasmic reticulum stress in endometrial cancer cells. J Cell Physiol 232(12):3735–3743. https://doi.org/10.1002/jcp.25850
Tian S, Wang X, Proud CG (2017) Oncogenic MNK signalling regulates the metastasis suppressor NDRG1. Oncotarget 8:46121–46135. https://doi.org/10.18632/oncotarget.17555
Tang Z, Qin S, Hao X, Zhu Z, Guanglin S, Caixin Z, Anaz M, Yi W, Songshi N, Xiaoyu Z (2018) Serum and glucocorticoid-regulated kinase 1 (SGK1) is a predictor of poor prognosis in non-small cell lung cancer, and its dynamic pattern following treatment with SGK1 inhibitor and gamma-ray irradiation was elucidated. Oncol Rep 39:1505–1515. https://doi.org/10.3892/or.2018.6181
Wang K, Gu S, Nasir O, Foller M, Ackermann TF, Klingel K, Kandolf R, Kuhl D, Stournaras C, Lang F (2010) SGK1-dependent intestinal tumor growth in APC-deficient mice. Cell Physiol Biochem 25:271–278. https://doi.org/10.1159/000276561
Abbruzzese C, Mattarocci S, Pizzuti L, Mileo AM, Visca P, Antoniani B, Gabriele A, Francesco F, Rosario A, Lucia D et al (2012) Determination of SGK1 mRNA in non-small cell lung cancer samples underlines high expression in squamous cell carcinomas. J Exp Clin Cancer Res 31:1–4. https://doi.org/10.1186/1756-9966-31-4
Chen X, Gu J, Wu Y, Liang P, Shen M, Xi J, Jian Q (2020) Clinical characteristics of colorectal cancer patients and anti-neoplasm activity of genistein. BioMed Pharmacother 124:109835. https://doi.org/10.1016/j.biopha.2020.109835
Feng Z, Liu L, Zhang C, Zheng T, Wang J, Lin M, Yuhan Z, Xiaowen W, Arnold JL, Wenwei H (2012) Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci USA 109:7013–7018. https://doi.org/10.1073/pnas.1203930109
Naruse T, Yanamoto S, Okuyama K, Yamashita K, Omori K, Nakao Y, Shin-Ichi Y, Masahiro U (2017) Therapeutic implication of mTORC2 in oral squamous cell carcinoma. Oral Oncol 65:23–32. https://doi.org/10.1016/j.oraloncology.2016.12.012
Wu W, Zou M, Brickley DR, Pew T, Conzen SD (2006) Glucocorticoid receptor activation signals through forkhead transcription factor 3a in breast cancer cells. Mol Endocrinol 20:2304–2314. https://doi.org/10.1210/me.2006-0131
Zhang Z, Xu Q, Song C, Mi B, Zhang H, Kang H, Huiyong L, Yunlong S, Jia W, Zhuowei L et al (2020) Serum- and glucocorticoid-inducible kinase 1 is essential for osteoclastogenesis and promotes breast cancer bone metastasis. Mol Cancer Ther 19:650–660. https://doi.org/10.1158/1535-7163.MCT-18-0783
Szmulewitz RZ, Chung E, Al-Ahmadie H, Daniel S, Kocherginsky M, Razmaria A (2012) Serum/glucocorticoid-regulated kinase 1 expression in primary human prostate cancers. Prostate 72:157–164. https://doi.org/10.1002/pros.21416
Ronchi CL, Sbiera S, Leich E, Tissier F, Steinhauer S, Deutschbein T, Martin F, Bruno A (2012) Low SGK1 expression in human adrenocortical tumors is associated with ACTH-independent glucocorticoid secretion and poor prognosis. J Clin Endocrinol Metab 97(12):E2251–E2260. https://doi.org/10.1210/jc.2012-2669
Sahoo S, Brickley DR, Kocherginsky M, Conzen SD (2005) Coordinate expressionbof the PI3-kinase downstream effectors serum and glucocorticoid-induced kinase (SGK-1) and Akt-1 in human breast cancer. Eur J Cancer 41:2754–2759. https://doi.org/10.1016/j.ejca.2005.07.018
Castel P, Haley E, Ruzica B, Eneda T, Pedram R, Javier C, Srinivasaraghavan K, Chandra SV, Maura D, Sarat C et al (2016) PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kalpha inhibition. Cancer Cell 30:229–242. https://doi.org/10.1016/j.ccell.2016.06.004
Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR (2013) Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J 452:499–508. https://doi.org/10.1042/BJ20130342
Sherk AB, Frigo DE, Schnackenberg CG, Bray JD, Laping NJ, Trizna W (2008) Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res 68:7475–7483. https://doi.org/10.1158/0008-5472.CAN-08-1047
Towhid ST, Liu GL, Ackermann TF, Beier N, Scholz W, Fuchß T, Mahmoud T, Hans-Peter R, Florian L (2013) Inhibition of colonic tumor growth by the selective SGK inhibitor EMD638683. Cell Physiol Biochem 32:838–848. https://doi.org/10.1159/000354486
Zhu J, Zhang R, Yang D, Li J, Yan X, Jin K, Li W, Liu X, Zhao J, Shang W et al (2018) Knockdown of long non-coding RNA XIST inhibited doxorubicin resistance in colorectal cancer by upregulation of miR-124 and downregulation of SGK1. Cell Physiol Biochem 51:113–128. https://doi.org/10.1159/000495168
D’Antona L, Dattilo V, Catalogna G, Scumaci D, Fiumara CV, Musumeci F, Giuseppe P, Silvia S, Rossana T, Cristina BS et al (2019) In preclinical model of ovarian cancer, the SGK1 inhibitor SI113 counteracts the development of paclitaxel resistance and restores drug sensitivity. Transl Oncol 12:1045–1055. https://doi.org/10.1016/j.tranon.2019.05.008
Abbruzzese C, Matteoni S, Persico M, Ascione B, Schenone S, Musumeci F, Rosario A, Nicola P, Paola M, Marco GP et al (2019) The small molecule SI113 hinders epithelial-to-mesenchymal transition and subverts cytoskeletal organization in human cancer cells. J Cell Physiol 234:22529–22542. https://doi.org/10.1016/j.tranon.2019.05.008
Liang X, Chunling L, Jinzhe Z, Wencheng F, Xuesha L, Yu A, Guanming J, Kejin W, Yongqin L, Jiahong X (2017) Development of a new analog of SGK1 inhibitor and its evaluation as a therapeutic molecule of colorectal cancer. J Cancer 8:2256–2262. https://doi.org/10.7150/jca.19566
Tangir J, Bonafe N, Gilmore-Hebert M, Henegariu O, Setsuko KC (2004) SGK1, a potential regulator of c-fms related breast cancer aggressiveness. Clin Exp Metastasis 21:477–483. https://doi.org/10.1007/s10585-004-4226-8
Fagerli UM, Ullrich K, Stuhmer T, Holien T, Kochert K, Holt RU, Bruland O, Chatterjee M, Nogai H, Lenz G et al (2011) Serum/glucocorticoid-regulated kinase 1 (SGK1) is a prominent target gene of the transcriptional response to cytokines in multiple myeloma and supports the growth of myeloma cells. Oncogene 30:3198–3206. https://doi.org/10.1038/onc.2011.79
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435. https://doi.org/10.1152/physrev.00030.2009
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477. https://doi.org/10.1016/s1534-5807(04)00099-1
Saxton RA, Sabatini DM (2017) mTOR signaling in growth, metabolism, and disease. Cell 169:361–371. https://doi.org/10.1016/j.cell.2017.02.004
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330:1344–1348. https://doi.org/10.1126/science.1193497
Jiao J, Demontis F (2017) Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr Opin Pharmacol 34:1–6. https://doi.org/10.1016/j.coph.2017.03.009
Li WW, Li J, Bao JK (2012) Microautophagy: lesser-known self-eating. Cell Mol Life Sci 69:1125–1136. https://doi.org/10.1007/s00018-011-0865-5
Yu L, Chen Y, Tooze SA (2018) Autophagy pathway: cellular and molecular mechanisms. Autophagy 14:207–215. https://doi.org/10.1080/15548627.2017.1378838
Mizushima N (2018) A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol 20(5):521–527. https://doi.org/10.1038/s41556-018-0092-5
Sosa MS, Bragado P, Aguirre-Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14(9):611–622. https://doi.org/10.1038/nrc3793
Luo L, Qin ZH (2019) Autophagy, aging, and longevity. Adv Exp Med Biol 1206:509–525. https://doi.org/10.1007/978-981-15-0602-4_24
Buytaert E, Callewaert G, Vandenheede JR, Agostinis P (2006) Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum. Autophagy 2:238–240. https://doi.org/10.4161/auto.2730
Bednarczyk M, Muc-Wierzgon M, Waniczek D, Fatyga E, Klakla K, Mazurek U, Wierzgon J (2017) Autophagy-related gene expression in colorectal cancer patients. J Biol Regul 31:923–927
Eissa S, Matboli M, Awad N, Kotb Y (2017) Identification and validation of a novel autophagy gene expression signature for human bladder cancer patients. Tumor Biol 39(4):1010428317698360. https://doi.org/10.1177/1010428317698360
Xu CX, Zhao L, Yue P, Fang G, Tao H, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY (2011) Augmentation of NVP-BEZ235’s anticancer activity against human lung cancer cells by blockage of autophagy. Cancer Biol Ther 12(6):549–555. https://doi.org/10.4161/cbt.12.6.16397
Yao Q, Chen J, Lv Y, Wang T, Zhang J, Fan J, Wang L (2011) The significance of expression of autophagy-related gene Beclin, Bcl-2, and Bax in breast cancer tissues. Tumor Biol 32(6):1163–1171. https://doi.org/10.1007/s13277-011-0219-9
Saha S, Panigrahi DP, Patil S, Sujit KB (2018) Autophagy in health and disease: a comprehensive review. Biomed Pharmacother 104:485–495. https://doi.org/10.1016/j.biopha.2018.05.007
Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C (2009) Autophagy suppresses tumorigenesis through elimination of p62. Cell 137:1062–1075. https://doi.org/10.1016/j.cell.2009.03.048
Mathew R, White E (2011) Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev 21:113–119. https://doi.org/10.1016/j.gde.2010.12.008
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402(6762):672–676. https://doi.org/10.1038/45257
Servante J, Estranero J, Meijer L, Layfield R, Grundy R (2018) Chemical modulation of autophagy as an adjunct to chemotherapy in childhood and adolescent brain tumors. Oncotarget 16:35266–35277. https://doi.org/10.18632/oncotarget.26186
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, Wang X, He C, Pan H (2013) Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4:e838. https://doi.org/10.1038/cddis.2013.350
Liu W, Xuchu W, Yiyun W, Yibei D, Yiyi X, Ying P, Binbin Y, Pan Y, Zhenping L, Xiuzhi D, Zhaoping L, Yuhua C, Chunhua L, Xiang L, Zhihua T (2018) SGK1 inhibition-induced autophagy impairs prostate cancer metastasis by reversing EMT. J Exp Clin Cancer Res 37(1):73. https://doi.org/10.1186/s13046-018-0743-1
Catalogna G, Talarico C, Dattilo V, Gangemi V, Calabria F, D’Antona L, Silvia S, Francesca M, Cataldo B, Nicola P et al (2017) The SGK1 kinase inhibitor SI113 sensitizes theranostic effects of the 64cucl2 in human glioblastoma multiforme cells. Cell Physiol Biochem 43:108–119. https://doi.org/10.1159/000480328
Zuleger T, Heinzelbecker J, Takacs Z, Catherine H, Jakob V, Florian L, Tassula PC (2018) SGK1 inhibits autophagy in murine muscle tissue. Oxid Med Cell Longev 2018:4043726. https://doi.org/10.1155/2018/4043726
Aspernig H, Heimbucher T, Qi W, Dipak G, Sedric C, Yijian Y, Erika DVG, Ralf B, Antje T (2019) Mitochondrial perturbations couple mTORC2 to autophagy in C. elegans. Cell Rep 29(6):399-1409.e5. https://doi.org/10.1016/j.celrep.2019.09.072
Maestro I, Patricia B, Ana M (2020) Serum- and glucocorticoid-induced kinase 1, a new therapeutic target for autophagy modulation in chronic diseases. Expert Opin Ther Targets 24(3):231–243. https://doi.org/10.1080/14728222.2020.1730328
Talarico C, Dattilo V, D’Antona L, Barone A, Amodio N, Belviso S, Francesca M, Claudia A, Cataldo B, Francesco T et al (2016) SI113, a SGK1 inhibitor, potentiates the effects of radiotherapy, modulates the response to oxidative stress and induces cytotoxic autophagy in human glioblastoma multiforme cells. Oncotarget 7:15868–15884. https://doi.org/10.18632/oncotarget.7520
Francipane MG, Lagasse E (2013) Selective targeting of human colon cancer stem-like cells by the mTOR inhibitor Torin-1. Oncotarget 4:1948–1962. https://doi.org/10.18632/oncotarget.1310
Berdel HO, Yin H, Liu JY, Grochowska K, Middleton C, Yanasak N, Abdelsayed R, Berdel WE, Mozaffari M, Yu JC et al (2014) Targeting serum glucocorticoid-regulated kinase-1 in squamous cell carcinoma of the head and neck: a novel modality of local control. PLoS ONE 9:e113795. https://doi.org/10.1371/journal.pone.0113795
Singh PK, Singh S, Ganesh S (2013) Activation of serum/glucocorticoid-induced kinase 1 (SGK1) underlies increased glycogen levels, mTOR activation, and autophagy defects in Lafora disease. Mol Biol Cell 24:3776–3786. https://doi.org/10.1091/mbc.E13-05-0261
Andres-Mateos E, Brinkmeier H, Burks TN, Mejias R, Files DC, Steinberger M, Arshia S, Ruth M, Jessica LS, Benjamin L et al (2013) Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol Med 5:80–91. https://doi.org/10.1002/emmm.201201443
Sang Y, Kong P, Zhang S, Zhang L, Cao Y, Duan X, Sun T, Tao Z, Liu W (2021) SGK1 in human cancer: emerging roles and mechanisms. Front Oncol 10:608722. https://doi.org/10.3389/fonc.2020.608722
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
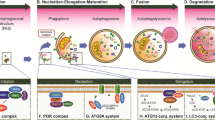
MJG conceptualized the study, searched the literature, drafted and reviewed the manuscript, and designed illustrations using BioRender tool.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghani, M.J. SGK1, autophagy and cancer: an overview. Mol Biol Rep 49, 675–685 (2022). https://doi.org/10.1007/s11033-021-06836-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06836-6