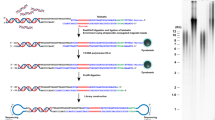
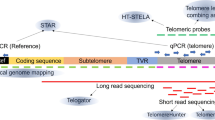
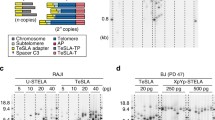
Abstract
Telomeres, guanine rich DNA sequences, which are found at both ends of human chromosomes, play a vital role in genome protection. These repetitive nucleotide sequences protect the genome from nucleolytic degradation, unnecessary recombination, and interchromosomal fusion. Though, as somatic cells go through replication cycles, their telomeres shrink until they reach a critical length called the Hayflick limit. At this limit, cellular senescence, an irreversible cell cycle arrest, is prompted. For all the above reasons, telomere length is a hopeful biomarker for age-associated diseases and cancer. While there are numerous methods for telomere measurement and quantification, there are still challenges for routine analysis in clinics as these methods are not simple and rapid. Recently, a new method has been developed that measures absolute length and absolute quantities of single telomere molecules. This method, single telomere absolute-length rapid (STAR) assay, which promises to measure telomere length rapidly and accurately, is also expected to be scalable. This review will discuss different telomere length measurement methods, including STAR assay, and will highlight each of their advantages and drawbacks. It will culminate in determining if STAR assay has the potential to be the superior method for telomere measurement.

Similar content being viewed by others
References
Ekundayo B, Bleichert F (2019) Origins of DNA replication. PLoS Genet 15(9):e1008320
Meselson M, Stahl FW (1958) The replication of DNA in Escherichia coli. Proc Natl Acad Sci U S A 44(7):671–682
Hanawalt PC (2004) Density matters: the semiconservative replication of DNA. Proc Natl Acad Sci U S A 101(52):17889–17894
Prescott DM, Kuempel PL (1972) Bidirectional replication of the chromosome in Escherichia coli. Proc Natl Acad Sci U S A 69(10):2842–2845
Wake RG (1972) Visualization of reinitiated chromosomes in Bacillus subtilis. J Mol Biol 68(3):501–509
Dewar JM, Walter JC (2017) Mechanisms of DNA replication termination. Nat Rev Mol Cell Biol 18(8):507–516
Duggin IG, Wake RG, Bell SD, Hill TM (2008) The replication fork trap and termination of chromosome replication. Mol Microbiol 70(6):1323–1333
Wynford-Thomas D, Kipling D (1997) Telomerase Cancer and the knockout mouse. Nature 389(6651):551–552
Olovnikov AM (1973) A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41(1):181–190
GM C, The Cell: A Molecular Approach. 2 ed. 2000
Pfeiffer V, Lingner J (2012) TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet 8(6):e1002747. https://doi.org/10.1371/journal.pgen.1002747
Zakian VA (2012) Telomeres: the beginnings and ends of eukaryotic chromosomes. Exp Cell Res 318(12):1456–1460
McClintock B (1941) The stability of broken ends of chromosomes in Zea Mays. Genetics 26(2):234–282
Blackburn EH, Greider CW, Szostak JW (2006) Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 12(10):1133–1138
Blackburn EH (1991) Structure and function of telomeres. Nature 350(6319):569–573
Zakian VA, Blanton HM (1988) Distribution of telomere-associated sequences on natural chromosomes in Saccharomyces cerevisiae. Mol Cell Biol 8(5):2257–2260
Blackburn EH (1984) The molecular structure of centromeres and telomeres. Annu Rev Biochem 53:163–194
Greider CW, Blackburn EH (1987) The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51(6):887–898
Takubo K, Nakamura K, Izumiyama N, Furugori E, Sawabe M, Arai T, Esaki Y, Mafune K, Kammori M, Fujiwara M, Kato M, Oshimura M, Sasajima K (2000) Telomere shortening with aging in human liver. J Gerontol A Biol Sci Med Sci 55(11):533–6
Jennings BJ, Ozanne SE, Hales CN (2000) Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol Genet Metab 71(1–2):32–42
Dunham MA, Neumann AA, Fasching CL, Reddel RR (2000) Telomere maintenance by recombination in human cells. Nat Genet 26(4):447–450
Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, Harley CB, Aviv A (2010) Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc 5(9):1596–1607
Aubert G, Hills M, Lansdorp PM (2012) Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat Res 730(1–2):59–67
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345(6274):458–460
Callicott RJ, Womack JE (2006) Real-time PCR assay for measurement of mouse telomeres. Comp Med 56(1):17–22 (PMID: 16521855)
Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, Raap AK, Tanke HJ (1996) Heterogeneity in telomere length of human chromosomes. Hum Mol Genet 5(5):685–691
Canela A, Vera E, Klatt P, Blasco MA (2007) High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A 104(13):5300–5305
Vera E, Blasco MA (2012) Beyond average: potential for measurement of short telomeres. Aging 4(6):379–392
Martens UM, Brass V, Engelhardt M, Glaser S, Waller CF, Lange W, Schmoor C, Poon SS, Landsdorp PM (2000) Measurement of telomere length in haematopoietic cells using in situ hybridization techniques. Biochem Soc Trans 28(2):245–250. https://doi.org/10.1042/bst0280245 (PMID: 10816136)
Baerlocher GM, Mak J, Tien T, Lansdorp PM (2002) Telomere length measurement by fluorescence in situ hybridization and flow cytometry: tips and pitfalls. Cytometry 47(2):89–99. https://doi.org/10.1002/cyto.10053 (PMID: 11813198)
Alter BP et al (2012) Telomere length is associated with disease severity and declines with age in dyskeratosis congenita”. Haematologica 97(3):353–359. https://doi.org/10.3324/haematol.2011.055269
Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM (1998) Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol 16(8):743–747. https://doi.org/10.1038/nbt0898-743 (PMID: 9702772)
Beh CW, Zhang Y, Zheng YL, Sun B, Wang TH (2018) Fluorescence spectroscopic detection and measurement of single telomere molecules. Nucleic Acids Res 46(19):e117
Nielsen PE, Egholm M (1999) An introduction to peptide nucleic acid. Curr Issues Mol Biol 1(1–2):89–104
Luo Y, Viswanathan R, Hande MP, Loh AHP, Cheow LF (2020) Massively parallel single-molecule telomere length measurement with digital real-time PCR. Sci Adv 6(34):eabb7944
Baird DM, Rowson J, Wynford-Thomas D, Kipling D (2003) Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet 33(2):203–207
Montpetit AJ, Alhareeri AA, Montpetit M, Starkweather AR, Elmore LW, Filler K, Mohanraj L, Burton CW, Menzies VS, Lyon DE, Jackson-Cook CK (2014) Telomere length: a review of methods for measurement. Nurs Res 63(4):289–299
Bendix L, Horn PB, Jensen UB, Rubelj I, Kolvraa S (2010) The load of short telomeres, estimated by a new method, Universal STELA, correlates with number of senescent cells. Aging Cell 9(3):383–397. https://doi.org/10.1111/j.1474-9726.2010.00568.x (Epub 2010 Mar 13 PMID: 20331440)
Lai TP, Zhang N, Noh J, Mender I, Tedone E, Huang E, Wright WE, Danuser G, Shay JW (2017) A method for measuring the distribution of the shortest telomeres in cells and tissues. Nat Commun 8(1):1356. https://doi.org/10.1038/s41467-017-01291-z
Lai TP, Simpson M, Patel K, Verhulst S, Noh J, Roche N, Heller D, Guirguis G, Shay JW, Herbig U, Aviv A (2021) Telomeres and replicative cellular aging of the human placenta and chorioamniotic membranes. Sci Rep 11(1):5115. https://doi.org/10.1038/s41598-021-84728-2
Kahl VFS et al (2020) Telomere length measurement by molecular combing. Fronti Cell Develop Biol 8:493. https://doi.org/10.3389/fcell.2020.00493
Lin J, Smith DL, Esteves K, Drury S (2019) Telomere length measurement by qPCR - Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 99:271–278
Author information
Authors and Affiliations
Contributions
AD prepared the manuscript and performed all revelant research toward to acquire all the facts. RM critically reviewed the paper and made necessary corrections and formatting.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dweck, A., Maitra, R. The advancement of telomere quantification methods. Mol Biol Rep 48, 5621–5627 (2021). https://doi.org/10.1007/s11033-021-06496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06496-6