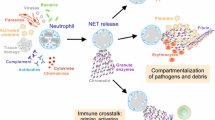
Abstract
Neutrophil extracellular traps (NETs) represent an innate organism defense mechanism characterized by neutrophil release of intracellular material to capture any aggressor agent. Elevated NETs release is associated with increased inflammatory response and related diseases, such as obesity. Chronic physical training is one of the main strategies to treat and prevent obesity. The relationship between physical training and NETs is still under study. The present review, followed by a bioinformatics analysis, demonstrates the meaningful connection between physical exercise, obesity, and NETs. The bioinformatics indicated TNF-α as a leading gene after the ontological analysis followed by positive-interleukin-6 regulation, chemokines, and inflammatory response regulation. The main results pointed to a relevant regulatory effect of physical training on NETs release, indicating physical exercise as a possible therapeutic target on modulating NETs and inflammation.
Graphical abstract







Similar content being viewed by others
References
Hawley JA, Hargreaves M, Joyner MJ, Zierath JR (2014) Integrative biology of exercise. Cell 159(4):738–749
Katzmarzyk PT, Janssen I (2004) The economic costs associated with physical inactivity and obesity in Canada: an update. Canadian Journal of Applied Physiology = Revue canadienne de physiologie appliquee 29(1):90–115
Gregg EW, Pereira MA, Caspersen CJ (2000) Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc 48(8):883–893
Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ, Program CCotNCE (2004) Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. J Am Coll Cardiol 44(3):720–732
Lautenschlager NT, Almeida OP (2006) Physical activity and cognition in old age. Curr Opin Psychiatry 19(2):190–193
Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB (2006) Daily activity energy expenditure and mortality among older adults. JAMA 296(2):171–179
Warburton DE, Nicol CW, Bredin SS (2006) Health benefits of physical activity: the evidence. Canadian Medical Association journal = journal de l’Association medicale canadienne 174(6):801–809
Gualano B, Sa Pinto AL, Perondi B, Leite Prado DM, Omori C, Almeida RT, Sallum AM, Silva CA (2010) Evidence for prescribing exercise as treatment in pediatric rheumatic diseases. Autoimmun Rev 9(8):569–573
Lima LCF, Saliba SW, Andrade JMO, Cunha ML, Cassini-Vieira P, Feltenberger JD, Barcelos LS, Guimarães ALS, De-Paula AMB, de Oliveira ACP et al (2017) Neurodegeneration alters metabolic profile and sirt 1 signaling in high-fat-induced obese mice. Mol neurobiol 54(5):3465–3475
Jorge ASB, Andrade JMO, Paraíso AF, Jorge GCB, Silveira CM, de Souza LR, Santos EP, Guimaraes ALS, Santos SHS, De-Paula AMB (2018) Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes Res Clin Pract 12(Suppl 2):1–8
Soares Crespo T, Oliveira Andrade JM, Barcala Jorge AS, de Paula AMB, Sena Guimarães AL, Sousa Santos SH (2016) Effects of omentectomy in addition to sleeve gastrectomy on the metabolic and inflammatory profiles of obese rats. Surg Obes Relat Dis 12(7):1292–1299
Freitas Lima LC, Braga VA, de Do Socorro FSM, Cruz JC, Sousa Santos SH, de OliveiraMonteiroBalarini MMCM (2015) Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol 6:304
Ciolac EG, Guimarães GV (2004) Exercício físico e síndrome metabólica. Revista Brasileira de Medicina do Esporte 10(4):319–324. https://doi.org/10.1590/S1517-86922004000400009
Lakka TA, Laaksonen DE, Lakka HM, Männikkö N, Niskanen LK, Rauramaa R, Salonen JT (2003) Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc 35(8):1279–1286
Dobbins M, Decorby K, Choi BC (2013) The association between obesity and cancer risk: a meta-analysis of observational studies from 1985 to 2011. ISRN Prev Med 2013:680536
Martin-Rodriguez E, Guillen-Grima F, Martí A, Brugos-Larumbe A (2015) Comorbidity associated with obesity in a large population: the APNA study. Obes Res Clin Pract 9(5):435–447
Wensveen FM, Valentić S, Šestan M, Turk Wensveen T, Polić B (2015) The “Big Bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol 45(9):2446–2456
Fruh SM (2017) Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract 29(S1):S3-s14
Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM (2010) Behavioral factors associated with successful weight loss after gastric bypass. Am Surg 76(10):1139–1142
Baar K (2006) Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc 38(11):1939–1944
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17(2):162–184
Fox EL, Bartels RL, Billings CE, Mathews DK, Bason R, Webb WM (1973) Intensity and distance of interval training programs and changes in aerobic power. Med Sci Sports 5(1):18–22
MacDougall JD, Hicks AL, MacDonald JR, McKelvie RS, Green HJ, Smith KM (1998) Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol 84(6):2138–2142
Macpherson RE, Hazell TJ, Olver TD, Paterson DH, Lemon PW (2011) Run sprint interval training improves aerobic performance but not maximal cardiac output. Med Sci Sports Exerc 43(1):115–122
da Silva FOC, Macedo DV (2011) Physical exercise, inflammatory process and adaptive condition: an overview. Braz J Kinanthropometry Hum Perform 13(4):320–328
Barnado A, Crofford LJ, Oates JC (2016) At the Bedside: neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J Leukoc Biol 99(2):265–278
Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, Choi JH, Choi Y, Shim S, Lyu IS et al (2017) Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun Rev 16(11):1160–1173
Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ, Wilson LD, Cooper DM (2006) Constitutive pro-and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol 100(4):1124–1133
Smith LL (2004) Tissue trauma: the underlying cause of overtraining syndrome? J Strength Cond Res 18(1):185–193
Shing CM, Peake J, Suzuki K, Okutsu M, Pereira R, Stevenson L, Jenkins DG, Coombes JS (2007) Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J Appl Physiol 102(3):1113–1122
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288(2):R345-353
Gleeson M (2006) Immune function in sport and exercise. Elsevier Health Sciences, Amsterdam
Speretta G, Leite R, Duarte A (2014) Obesidade, inflamação e exercício: foco sobre o TNF-alfa e IL-10. Revista Hospital Universitário Pedro Ernesto (TÍTULO NÃO-CORRENTE) 13(1). https://doi.org/10.12957/rhupe.2014.9807
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA (2011) The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11(9):607–615
Beavers KM, Brinkley TE, Nicklas BJ (2010) Effect of exercise training on chronic inflammation. Clin Chim Acta 411(11–12):785–793
Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP (2001) Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol 153(3):242–250
Pedersen BK (2011) Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun 25(5):811–816
Oh EG, Bang SY, Kim SH, Hyun SS, Chu SH, Jeon JY, Im JA, Lee JE, Lee MK (2013) Therapeutic lifestyle modification program reduces plasma levels of the chemokines CRP and MCP-1 in subjects with metabolic syndrome. Biol Res Nurs 15(1):48–55
Trøseid M, Lappegård KT, Claudi T, Damås JK, Mørkrid L, Brendberg R, Mollnes TE (2004) Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J 25(4):349–355
Shephard RJ, Shek PN (1994) Potential impact of physical activity and sport on the immune system—a brief review. Br J Sports Med 28(4):247–255
Smith JA, Telford RD, Mason IB, Weidemann MJ (1990) Exercise, training and neutrophil microbicidal activity. Int J Sports Med 11(3):179–187
Dufaux B, Order U (1989) Plasma elastase-α1-antitrypsin, neopterin, tumor necrosis factor, and soluble interleukin-2 receptor after prolonged exercise. Int J Sports Med 10(06):434–438
Czaikoski PG, Mota JM, Nascimento DC, Sônego F, Castanheira FV, Melo PH, Scortegagna GT, Silva RL, Barroso-Sousa R, Souto FO et al (2016) Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE 11(2):e0148142
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535
Zawrotniak M, Rapala-Kozik M (2013) Neutrophil extracellular traps (NETs)—formation and implications. Acta Biochim Pol 60(3):277–284
Rodrigues HG, Takeo Sato F, Curi R, Vinolo MAR (2016) Fatty acids as modulators of neutrophil recruitment, function and survival. Eur J Pharmacol 785:50–58
Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, Piconese S, Parenza M, Guiducci C, Vitali C (2012) Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood J Am Soc Hematol 120(15):3007–3018
Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD (2012) Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci 109(32):13076–13081
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13(3):159–175
Beiter T, Fragasso A, Hudemann J, Schild M, Steinacker J, Mooren FC, Niess AM (2014) Neutrophils release extracellular DNA traps in response to exercise. J Appl Physiol 117(3):325–333
de Oliveira PF, Farias LC, de Carvalho Fraga CA, Bambirra W Jr, Brito-Júnior M, Sousa-Neto MD, Santos SHS, de Paula AMB, D’Angelo MFSV, Guimarães ALS (2015) Bioinformatics, interaction network analysis, and neural networks to characterize gene expression of radicular cyst and periapical granuloma. J Endod 41(6):877–883
Barone A, Toti P, Giuca MR, Derchi G, Covani U (2015) A gene network bioinformatics analysis for pemphigoid autoimmune blistering diseases. Clin Oral Invest 19(6):1207–1222
Rebhan M, Chalifa-Caspi V, Prilusky J, Lancet D (1997) GeneCards: integrating information about genes, proteins and diseases. Trends in Genet 13(4):163
Bragazzi NL, Sivozhelezov V, Nicolini C (2011) Leader gene: a fast data-mining tool for molecular genomics. J Proteomics Bioinform 4(4):083–086
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176(2):231–241
Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J, Barthwal MK, Dikshit M (2012) Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE 7(10):e48111
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191(3):677–691
Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, Verma A, Mitra K, Barthwal MK, Krishnamurthy H et al (2010) Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide 22(3):226–234
Pattison DI, Davies MJ (2006) Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem 13(27):3271–3290
Dinarello CA (1997) Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112(6 Suppl):321S-329S
Fortin CF, McDonald PP, Fulop T, Lesur O (2010) Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock 33(4):344–352
Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM (2007) Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 49(1):88–98 (98 e81–82)
Wang H, Wang C, Zhao MH, Chen M (2015) Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol 181(3):518–527
Ma Y-H, Ma T-T, Wang C, Wang H, Chang D-Y, Chen M, Zhao M-H (2016) High-mobility group box 1 potentiates antineutrophil cytoplasmic antibody-inducing neutrophil extracellular traps formation. Arthritis Res Ther 18(1):2
Berezin A (2016) The neutrophil extracellular traps: the missed link between microvascular inflammation and diabetes. Metabolomics 6(163):2153–0769 (100016)
Zicker MC, Silveira ALM, Lacerda DR, Rodrigues DF, Oliveira CT, de Souza Cordeiro LM, Lima LCF, Santos SHS, Teixeira MM, Ferreira AVM (2019) Virgin coconut oil is effective to treat metabolic and inflammatory dysfunction induced by high refined carbohydrate-containing diet in mice. J Nutr Biochem 63:117–128
Jorge AS, Jorge GC, Paraíso AF, Franco RM, Vieira LJ, Hilzenderger AM, Guimarães AL, Andrade JM, De-Paula AM, Santos SH (2017) Brown and white adipose tissue expression of IL6, UCP1 and SIRT1 are associated with alterations in clinical, metabolic and anthropometric parameters in obese humans. Exp Clin Endocrinol Diabetes 125(3):163–170
Pinheiro TA, Barcala-Jorge AS, Andrade JMO, Pinheiro TA, Ferreira ECN, Crespo TS, Batista-Jorge GC, Vieira CA, Lelis DF, Paraíso AF et al (2017) Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J Nutr Biochem 48:74–82
Freire RH, Fernandes LR, Silva RB, Coelho BS, de Araújo LP, Ribeiro LS, Andrade JM, Lima PM, Araújo RS, Santos SH et al (2016) Wheat gluten intake increases weight gain and adiposity associated with reduced thermogenesis and energy expenditure in an animal model of obesity. Int J Obes 40(3):479–486
Nimmo M, Leggate M, Viana J, King J (2013) The effect of physical activity on mediators of inflammation. Diabetes Obes Metab 15(s3):51–60
Lira FS, Rosa JC, Pimentel GD, Tarini VA, Arida RM, Faloppa F, Alves ES, Do Nascimento CO, Oyama LM, Seelaender M et al (2010) Inflammation and adipose tissue: effects of progressive load training in rats. Lipids Health Dis 9:109
Wang J, Polaki V, Chen S, Bihl JC (2020) Exercise improves endothelial function associated with alleviated inflammation and oxidative stress of perivascular adipose tissue in type 2 diabetic mice. Oxid Med Cell Longev 2020:8830537
Smith TTG, Barr-Gillespie AE, Klyne DM, Harris MY, Amin M, Paul RW, Cruz GE, Zhao H, Gallagher S, Barbe MF (2020) Forced treadmill running reduces systemic inflammation yet worsens upper limb discomfort in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord 21(1):57
Silva J, Stone W, Honorato FS, Deus LA, Prestes J, Simões HG, Vieira EC, de Melo GF, Moraes MR, Rosa TS (2020) Resistance training improves sleep quality, redox balance and inflammatory profile in maintenance hemodialysis patients: a randomized controlled trial. Sci Rep 10(1):11708. https://doi.org/10.1038/s41598-020-68602-1
Shokouhi G, Ahmadiasl N, Roshangar L, Ghorbanihaghjo A, Sheikhzadeh F, Mesgari M, Kosari-Nasab M (2020) Long term treadmill exercise affects age-related oxidative stress in the spinal cord of rats. Comp Exerc Physiol. https://doi.org/10.3920/CEP200031
Funding
This work was partially supported by grants from Coordenadoria de Aperfeiçoamento do Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Contributions
BS: conceptualization, methodology, writing—original draft. DF, RMJ, IM, JS, VG: writing—original draft. SS: conceptualization, writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Consent to submit has been received explicitly from all co-authors, as well as from the responsible authorities—tacitly or explicitly—at the institute/organization where the work has been carried out, before the work is submitted.
Consent to publish
The Author transfers to Molecular Biology Reports the publication rights and he warrants that his/her contribution is original and that he/she has full power to make this grant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Valeria Oliveira de Sousa, B., de Freitas, D.F., Monteiro-Junior, R.S. et al. Physical exercise, obesity, inflammation and neutrophil extracellular traps (NETs): a review with bioinformatics analysis. Mol Biol Rep 48, 4625–4635 (2021). https://doi.org/10.1007/s11033-021-06400-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06400-2