Abstract
Capsicum as a spice crop, has wild and cultivated forms admired globally, including Indian subcontinent with vast climatic ranges. Systematic representation of the Indian Capsicum is required to address species relationships and sustainable agriculture, in face of unpredictable climatic conditions. We have updated the catalogue of Indian ‘C. annuum complex’ with 28 landraces and populations from different agro-climatic regions. The agro-climatic influence on the origin of stable chili landraces in India is remarkable, especially in the North East. The floral and fruit morphotype standards and chromosomal attributes have been considered for four distinct ‘C. annuum complex’ members under three species. The highlights of study are: (1) comparative profiling of Indian Capsicum species revealing less infraspecific variation within C. frutescens and C. chinense than C. annuum, at par with cultivation status, (2) karyotype analysis of some unique diploid landraces of C. annuum, (3) karyotypic confirmation of the polyploid Dalle Khursani landraces exclusive to India. To obtain more information, we attempted to correlate diversity of fruit and floral morphotype with chromosomal diversity. Existence of elite and rare germplasm found in the regional pockets offer great scope for enriching the agricultural tradition. The present dataset may serve as a template to be continuously upgraded by taxonomists, genomicists and breeders.






Similar content being viewed by others
References
Wall MM, Bosland PW (1998) Analytical methods for color and pungency of chiles (capsicums). Developments in food science. Elsevier, Amsterdam, pp 347–373
Walsh BM, Hoot SB (2001) Phylogenetic relationships of Capsicum (Solanaceae) using DNA sequences from two noncoding regions: the chloroplast atpB-rbcL spacer region and nuclear waxy introns. Int J Plant Sci 162:1409–1418
Jarret RL, Barboza GE, da Costa Batista FR, Berke T, Chou YY, Hulse-Kemp A, Ochoa-Alejo N, Tripodi P, Veres A, Garcia CC, Csillery G (2019) Capsicum—an abbreviated compendium. J Am Soc Hortic Sci 144:3–22
Perry L, Dickau R, Zarrillo S, Holst I, Pearsall DM, Piperno DR, Berman MJ, Cooke RG, Rademaker K, Ranere AJ, Raymond JS (2007) Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 315:986–988
Bosland PW, Votava EJ (2012) Seeds. Peppers: vegetable and spice capsicums edition 2. CAB International Publishing, New York, pp 55–65
Noss CF, Levey DJ (2014) Does gut passage affect post-dispersal seed fate in a wild chili, Capsicum annuum? Southeast Nat 13:475–483
Carrizo García C, Barfuss MH, Sehr EM, Barboza GE, Samuel R, Moscone EA, Ehrendorfer F (2016) Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann Bot 118:35–51
Perry L, Flannery KV (2017) Precolumbian use of chili peppers in the valley of Oaxaca, Mexico. Proc Natl Acad Sci 104:11905–11909
Andrews J (1995) Peppers: the domesticated Capsicums. University of Texas Press, Austin
Dhaliwal MS (2007) Solanaceous vegetables. Handbook of vegetable crops, Kalyani Publishers, Ludhiana, pp 34–76
Tripodi P, Kumar S (2019) The capsicum crop: an introduction. In: Kole C, Ramchiary N (eds) The Capsicum genome. Springer, Cham, pp 1–8
Barboza GE, Carrizo García C, Leiva González S, Scaldaferro M, Reyes X (2019) Four new species of Capsicum (Solanaceae) from the tropical Andes and an update on the phylogeny of the genus. PLoS ONE 14:e0209792
Bosland PW, Votava EJ (2000) Peppers–vegetable and spice Capsicum. Crop production science in horticulture. CAB International Publishing, New York
Park YK, Park KC, Park CH, Kim NS (2000) Chromosomal localization and sequence variation of 5S rRNA gene in five Capsicum species. Mol Cells 10:18–24
Baral JB, Bosland PW (2004) Unraveling the species dilemma in Capsicum frutescens and C. chinense (Solanaceae): a multiple evidence approach using morphology, molecular analysis, and sexual compatibility. J Am Soc Hortic Sci 129:826–832
Jarret RL, Dang P (2004) Revisiting the waxy locus and the Capsicum annuum L. complex. Ga J Sci 62:118
Ryzhova NN, Kochieva EZ (2004) Analysis of microsatellite loci of the chloroplast genome in the genus Capsicum (pepper). Russ J Genet 40:892–896
Pickersgill B (1988) The genus Capsicum: a multidisciplinary approach to the taxonomy of cultivated and wild plants. Biol Zent 107:381–389
González-Pérez S, Garcés-Claver A, Mallor C, de Miera LE, Fayos O, Pomar F, Merino F, Silvar C (2014) New insights into Capsicum spp relatedness and the diversification process of Capsicum annuum in Spain. PLoS ONE 9:e116276
Baral JB, Bosland PW (2002) Genetic diversity of a Capsicum germplasm collection from Nepal as determined by randomly amplified polymorphic DNA markers. J Am Soc Hortic Sci 127:316–324
Bosland PW, Baral JB (2007) ‘Bhut Jolokia’—the world’s hottest known chile pepper is a putative naturally occurring interspecific hybrid. Hortic Sci 42:222–224
Ravishankar GA, Suresh B, Giridhar P, Rao SR, Johnson TS (2003) Biotechnological studies on Capsicum for metabolite production and plant improvement. In: De AK (ed) Capsicum: the genus Capsicu. CRC Press, Harwood Academic Publishers, Boca Raton, pp 96–128
Reddy MK, Srivastava A, Kumar S, Kumar R, Chawda N, Ebert AW, Vishwakarma M (2014) Chili (Capsicum annuum L.) breeding in India: an overview. SABRAO J Breed Genet 46:160–173
Hunziker AT (1979) South American Solanaceae: a synoptic survey. In: Hawkes J, Glester RN, Skelding AD (eds) The biology and taxonomy of the Solanacea. Academic Press, London, pp 49–85
Moscone EA, Scaldaferro MA, Grabiele M, Cecchini NM, Sánchez García Y, Jarret R, Daviña JR, Ducasse DA, Barboza GE, Ehrendorfer F (2007) The evolution of chili peppers (Capsicum-Solanaceae): a cytogenetic perspective. In: Spooner DM et al. (eds) VI’ International Solanaceae Conference. Acta Hortic 745:137–170
Ince AG, Karaca M, Onus AN (2010) Genetic relationships within and between Capsicum species. Biochem Genet 48:83–95
Zhigila DA, Abdul Rahaman AA, Kolawole OS, Oladele FA (2014) Fruit morphology as taxonomic features in five varieties of Capsicum annuum L Solanaceae. J Bot 2014:1–6
Jha TB, Saha PS (2017) Characterization of some Indian Himalayan Capsicums through floral morphology and EMA-based chromosome analysis. Protoplasma 254:921–933
Wang D, Bosland PW (2006) The genes of Capsicum. Hortic Sci 41:1169–1187
van Zonneveld M, Ramirez M, Williams DE, Petz M, Meckelmann S, Avila T, Bejarano C, Ríos L, Peña K, Jäger M, Libreros D (2015) Screening genetic resources of Capsicum peppers in their primary center of diversity in Bolivia and Peru. PLoS ONE 10:e0134663
Moreira AF, Ruas PM, de Fátima RC, Baba VY, Giordani W, Arruda IM, Rodrigues R, Gonçalves LSA (2018) Genetic diversity, population structure and genetic parameters of fruit traits in Capsicum chinense. Sci Hortic 236:1–9
Igwe DO, Afiukwa CA, Acquaah G, Ude GN (2019) Genetic diversity and structure of Capsicum annuum as revealed by start codon targeted and directed amplified minisatellite DNA markers. Hereditas 156:32
Alvares Bianchi P, Renata Almeida da Silva L, André da Silva Alencar A, Henrique Araújo Diniz -Santos P, Pimenta S, Pombo Sudré C, Corte ED, Simões Azeredo Gonçalves L, Rodrigues R (2020) Biomorphological characterization of Brazilian Capsicum chinense Jacq germplasm. Agronomy 10:447
Lee HY, Ro NY, Patil A, Lee JH, Kwon JK, Kang BC (2020) Uncovering candidate genes controlling major fruit-related traits in pepper via genotype-by-sequencing based QTL mapping and genome-wide association study. Front Plant Sci 11:1100
Guerra M (2008) Chromosome numbers in plant cytotaxonomy: concepts and implications. Cytogenet Genom Res 120:339–350
Soza VL, Haworth KL, Di Stilio VS (2013) Timing and consequences of recurrent polyploidy in meadow-rues (Thalictrum, Ranunculaceae). MBE 30:1940–1954
Soltis DE, Visger CJ, Soltis PS (2014) The polyploidy revolution then and now: Stebbins revisited. Am J Bot 101:1057–1078
Mota L, Torices R, Loureiro J (2016) The evolution of haploid chromosome numbers in the sunflower family. GBE 8:3516–3528
Viruel J, Kantar MB, Gargiulo R, Hesketh-Prichard P, Leong N, Cockel C, Forest F, Gravendeel B, Pérez-Barrales R, Leitch IJ, Wilkin P (2021) Crop wild phylorelatives (CWPs): phylogenetic distance, cytogenetic compatibility and breeding system data enable estimation of crop wild relative gene pool classification. Bot J Linn Soc 195:1–33
Weiss-Schneeweiss H, Schneeweiss GM (2013) Karyotype diversity and evolutionary trend in angiosperms. In: Leitch IJ, Greilhuber J, Dolezel J, Wendel JF (eds) Plant genome diversity physical structure, behavior and evolution of plant genomes, vol 2. Springer Verlag, Vienna, pp 209–230
Deakin JE, Potter S, O’Neill R, Ruiz-Herrera A, Cioffi MB, Eldridge MD, Fukui K, Marshall Graves JA, Griffin D, Grutzner F, Kratochvíl L (2019) Chromosomics: bridging the gap between genomes and chromosomes. Genes 10:627
Zhang P, Friebe B, Gill B, Park RF (2007) Cytogenetics in the age of molecular genetics. Aust J Agric Res 58:498–506
Liehr T (2017) Classical cytogenetics” is not equal to “banding cytogenetics. Mol Cytogenet 10:3. https://doi.org/10.1186/s13039-017-0305-9
Pickersgill B (1997) Genetic resources and breeding of Capsicum. Euphytica 96:129–133
Moscone EA, Lambrou M, Hunziker AT, Ehrendorfer F (1993) Giemsa C- banded karyotypes in Capsicum (Solanaceae). Plant Syst Evol 186:213–229
Moscone EA, Lambrou M, Ehrendorfer F (1996) Fluorescent chromosome banding in the cultivated species of Capsicum (Solanaceae). Plant Syst Evol 202:37–63
Moscone EA, Scaldaferro MA, Grabiele M, Romero MV, Debat H, Seijo JG, Acosta MC, Davina JR, Barboza GE, Ducasse DA (2011) Genomic characterization of the chili peppers (Capsicum, Solanaceae) germplasm by classical and molecular cytogenetics. Physical mapping technologies for the identification and characterization of mutated genes contributing to crop quality. International Atomic Energy Agency, Vienna, pp 97–104
Pozzobon MT, Schifino-Wittmann MT (2006) A meiotic study of the wild and semi-domesticated Brazilian species of genus Capsicum L. (Solanaceae). Cytologia 71:275–287
Pozzobon MT, Schifino-Wittniann MT, Bianchetti LB (2006) Chromosome numbers in wild and semidomesticated Brazilian Capsicum L. (Solanaceae) species: do x= 12 and x= 13 represent two evolutionary lines? Bot J Linn Soc 151:259–269
Scaldaferro MA, Seijo JG, Acosta MC, Barboza GE, Ducasse DA, Moscone EA (2006) Genomic characterization of the germplasm in peppers (Capsicum-Solanaceae) by fluorescent in situ hybridization. Plant Sci 43:291–297
Scaldaferro MA, Grabiele M, Moscone EA (2013) Heterochromatin type, amount and distribution in wild species of chili peppers (Capsicum, Solanaceae). Genet Resour Crop Evol 60:693–709
Scaldaferro MA, da Cruz MVR, Cecchini NM, Moscone EA (2016) FISH and AgNOR mapping of the 45S and 5S rRNA genes in wild and cultivated species of Capsicum (Solananceae). Genome 59:95–113
Scaldaferro MA, Moscone EA (2019) Cytology and DNA content variation of Capsicum genomes. In: Ramchiary N, Kole C (eds) The Capsicum genome. Springer, Cham, pp 57–84
Mathur R, Dangi RS, Dass SC, Malhotra RC (2000) The hottest chilli variety in India. Curr Sci 79:287–288
Misra AK, Manohar SS, Kumar A (2007) Characterization of indigenously collected germplasm of yellow sarson (B. rapa L. var. yellow sarson) for yield contributing traits. In: Tingdong FU, Chunyun G (eds) Proceedings of the 12th International Rapeseed Congress I. Genetics and Breeding. Science Press USA Inc, pp 280–283
Singh RJ (2016) Plant cytogenetics. CRC Press, Boca Raton
IPGRI, AVRDC, CATIE (1995) Descriptors for Capsicum (Capsicum spp.). International Plant Genetic Resources Institute, Rome, Italy; the Asian Vegetable Research and Development Center, Taipei, Taiwan, and the Centro Agronómico Tropical de Investigación y Enseñanza, Turrialba, Costa Rica. ISBN 92-9043-216-0
Jha TB, Dafadar A, Ghoroi A (2012) New genetic resource in Capsicum L. from Eastern Himalayas. Plant Genet Resour 10:141–144
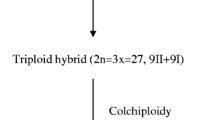
Jha TB, Saha PS, Nath S, Das A, Jha S (2017) Morphological and cytogenetical characterization of ‘Dalle Khursani’: a polyploid cultivated Capsicum of India. Sci Hortic 215:80–90
do Rêgo ER, do Rêgo MM (2016) Genetics and breeding of chili pepper Capsicum spp. In: do Rêgo ER, do Rêgo MM, Finger FL (eds) Production and breeding of chilli peppers (Capsicum spp). Springer International Publishing, Switzerland, pp 57–80
Pinto CMF, dos Santos IC, de Araujo FF, da Silva TP (2016) Pepper importance and growth (Capsicum spp). In: do Rego ER, do Rêgo MM, Finger FL (eds) Production and breeding of chilli peppers (Capsicum spp.). Springer, Switzerland, pp 1–25
Pickersgill B (1984) Migration of chili peppers, Capsicum spp, in the Americas. In: Stone D (ed) Pre-Columbian plant migration. 631.5098 St711p Ej. 1
Bosland PW (1993) An effective plant field cage to increase the production of genetically pure chile (Capsicum spp.) seed. HortScience 28:1053–1053
Onus AN, Pickersgill B (2004) Unilateral incompatibility in Capsicum (Solanaceae): occurrence and taxonomic distribution. Ann Bot 94:289–295
Bhutia KL, Bhutia ND, Khanna VK (2019) Rich genetic diversity of Capsicum species in Northeast India, as a potential source for chilli crop improvement. J Agric For Meteorol Res 2:77–83
González-Salán MM, Bosland PW (1991) Sources of resistance to Verticillium wilt in Capsicum. Euphytica 59:49–53
Huskins CL, La-Cour L (1930) Chromosome numbers in Capsicum. Am Nat 64:382–384
Dixit PD (1931) A cytological study of Capsicum annuum. Indian J Agric Sci 1:419–433
Votava EJ, Baral JB, Bosland PW (2005) Genetic diversity of chile (Capsicum annuum var. annuum L.) landraces from northern New Mexico, Colorado, and Mexico. Econ Bot 59:8–17
Castañón-Nájera G, Latournerie-Moreno L, Mendoza-Elos M, Vargas-López A, Cárdenas-Morales H (2008) Collection and characterization of chili (Capsicum spp.) in Tabasco, Mexico. Phyton 77:189–202
Pérez Castañeda LM, Castañón Nájera G, Mayek Pérez N (2008) Morphological diversity of chili peppers (Capsicum spp.) from Tabasco, Mexico. Biodivers Noteb 27:11–22
Moscone EA (1990) Chromosome studies on Capsicum (Solanaceae) I. Karyotype analysis in C chacoënse. Brittonia 42:147–154
Lambrou M, Hunziker AT, Ehrendorfer F (1993) Giemsa C-banded karyotypes in Capsicum (Solanaceae). Plant Syst Evol 186:213–229
Lanteri S, Pickersgill B (1993) Chromosomal structural changes in Capsicum annuum L and C. chinense Jac. Euphytica 67:155–160
Loidl J, Ehrendorfer F, Hunziker AT (1995) Analysis of active nucleolus organizing regions in Capsicum (Solanaceae) by silver staining. Am J Bot 82:276–287
Rohami M, Mohammadi A, Mahmood K, Ahmadi H, Daraneh N (2010) Karyotype analysis of several ecotypes of Capsicum annuum L. in Iran. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 38:177–180
Barboza GE, Agra MF, Romero MV, Scaldaferro MA, Moscone EA (2011) New endemic species of Capsicum (Solanaceae) from the Brazilian Caatinga: comparison with the re-circumscribed C. parvifolium. Syst Bot 36:768–781
Ibiza VP, Blanca J, Cañizares J, Nuez F (2012) Taxonomy and genetic diversity of domesticated Capsicum species in the Andean region. Genet Resour Crop Evol 59:1077–1088
Sousa WRDN, Lopes ACDA, Carvalho RD, Gomes RLF, Peron AP (2015) Karyotypic characterization of Capsicum sp. accessions. Acta Sci Agron 37:147–153
Romero-da Cruz MV, Urdampilleta JD, Forni Martins ER, Moscone EA (2017) Cytogenetic markers for the characterization of Capsicum annuum L. cultivars. Plant Biosyst 151:84–91
Souza-Macedo VD, García-Dávila MA, Castro GR, Garzón-Bautista YM, Caetano CM (2017) Cytogenetic evaluation of chili (Capsicum spp., Solanaceae) genotypes cultivated in Valle del Cauca, Colombia. Acta Agronómica 66:612–617
Grabiele M, Debat HJ, Scaldaferro MA, Aguilera PM, Moscone EA, Seijo JG, Ducasse DA (2018) Highly GC-rich heterochromatin in chili peppers (Capsicum-Solanaceae): a cytogenetic and molecular characterization. Sci Hortic 238:391–399
Raghavan TS, Venkatasubban KP (1940) Studies in the S. Indian chillies I. Proc Indian Acad Sci 12:29–46
Chennaveeraiah MS, Habib AF (1966) Recent advances in cytogenetics of Capsicums. Proc Autumn Sch Bot, pp 69–90
Datta PC (1968) Karyology of Indian varieties of Capsicum annuum Linn. (Solanaceae). Caryologia 21:121–126
Chennaveeraiah MS, Habib AF (1973) Cytomorphological studies in a spontaneous triploid of Capsicum annuum L. Cytologia 38:677–685
Reddi MV, Rao GM (1974) Somatic karyotype and chiasma frequency in Capsicum annuum L. Cytologia 39:581–584
Limaye VA, Patil VP (1989) Karyomorphological studies in the genus Capsicum Linn. Cytologia 54:455–463
Neeti C, Verma S, Sharma N (2010) Genetic Systems in chillies II. Meiotic System of three cultivars of Capsicum annuum L. Caryologia 63:1–10
Cheema SK, Pant MR (2013) Karyotype analysis of seven cultivated varieties of Capsicum annuum L. Caryologia 66:70–75
Haque SM, Paul S, Ghosh B (2016) Karyological studies of two hot chilli pepper cultivars from two different geographical regions of India: Bhut jolokia, Capsicum chinense Jacq. and Bullet Lanka Capsicum annuum L. Nucleus 59:227–233
Jha TB, Bhowmick BK (2021) Unravelling the genetic diversity and phylogenetic relationships of Indian Capsicum through fluorescent banding. Genet Resour Crop Evol 68:205–225
Phulari SS, Dixit GB (1996) Cytological studies in Capsicum. I. Karyomorphological studies in parents and hybrids of Capsicum annuum and C. frutescens. Adv Plant Sci 9:111–119
Fukui K (1996) Plant chromosomes at mitosis. In: Fukui K, Nakayama S (eds) Plant chromosomes: laboratory methods. CRC Press Inc, Boca Raton, pp 1–17
Kurata N, Omura T (1978) Karyotype analysis in rice. 1. A new method for identifying all chromosome pairs. Jpn J Genet 53:251–255
Fukui K, Iijima K (1991) Somatic chromosome map of rice by imaging methods. Theor Appl Genet 81:589–596
Yamamoto M, Takada N, Hirabayashi T, Kubo T, Tominaga S (2010) Fluorescent staining analysis of chromosomes in pear (Pyrus spp.). J Jpn Soc Hortic Sci 79:23–26
Bhowmick BK, Jha TB, Jha S (2012) Chromosome analysis in the dioecious cucurbit Coccinia grandis (L.) Voigt. Chrom Sci 15:9–15
Jha TB, Halder M (2015) Searching chromosomal landmarks in Indian lentils through EMA based Giemsa staining method. Protoplasma 253:1223–1231
Bhowmick BK, Jha S (2015) Differential heterochromatin distribution, flow cytometric genome size and meiotic behavior of chromosomes in three Cucurbitaceae species. Sci Hortic 193:322–329
Bhowmick BK, Jha S (2019) Differences in karyotype and fluorochrome banding patterns among variations of Trichosanthes cucumerina with different fruit size. Cytologia 84:1–10
Ghosh I, Bhowmick BK, Jha S (2018) Cytogenetics of two Indian varieties of Momordica charantia L. (bittergourd). Sci Hortic 240:333–343
Jha TB (2019) Karyotype analysis from aerial roots of Piper nigrum based on Giemsa and fluorochrome banding. Cytologia 84:313–317
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditus 52:210–220
Harrison CJ, Alvey E, Henderson IR (2010) Meiosis in flowering plants and other green organisms. J Exp Bot 61:2863–2875
Lavinscky MP, Souza MM, Silva GS, Melo CAF (2017) Contributions of classical and molecular cytogenetic in meiotic analysis and pollen viability for plant breeding. Genet Mol Res. https://doi.org/10.4238/gmr16039582
Sharma AK, Sharma A (1980) Chromosome techniques. Theory and practice, 3rd edn. Butterworth and Co, London
Pickersgill B (1977) Chromosomes and evolution in Capsicum. Capsicum 77:27–37
Martins KC, Pereira TNS, Souza SAM, Rodrigues R, Amaral Junior ATD (2015) Crossability and evaluation of incompatibility barriers in crosses between Capsicum species. Crop Breed Appl Biotechnol 15:139–145
da Costa Batista FR (2016) Cytogenetics in Capsicum L. In: do Rêgo ER, do Rêgo MM, Finger FL (eds) Production and breeding of chilli peppers (Capsicum spp. Springer International Publishing, Switzerland, pp 41–56
Acknowledgements
TBJ acknowledges the Principal, Dr. S. Dutta and Head, Dept. of Botany, Maulana Azad College for providing basic facilities.
Author information
Authors and Affiliations
Contributions
TJ and BKB equally contributed to writing the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1
(TIF 6283 kb). Comparative plant habits of some less cultivated diploid C. annuum (2n=24) landraces. a Karenga, b Kul (arrow points to inset showing enlarged fruit), c Kalo [arrow points to inset showing leaves of Aakashi (left) and Kalo (right)], d. Sada, e. Round (arrow points to inset showing fruit), f Ghee, g Dhani type I (Jalpaiguri), h Naga jolokia type I (Dibrugarh)
Supplementary file2
(TIF 12058 kb). Plant habits of polyploid (2n=48) C. annuum Dalle Khursani. a type III (Mungpoo), b type I (Darjeeling), c type VII (Jungi) with inset showing flowers and fruits, d type V (Takdah). e–h Leaves of Dalle Khursani populations, e type IV (Kolakham), f type VIII (Sikkim) in comparison with leaf of diploid Aakashi at right, g type VII (Jungi), h Dentate calyx margin and annular constriction at base of calyx (indicated by arrows) in Dalle Khursani flower (type IV, Kolakham). Bar 1cm
Rights and permissions
About this article
Cite this article
Jha, T.B., Bhowmick, B.K. Conservation of floral, fruit and chromosomal diversity: a review on diploid and polyploid Capsicum annuum complex in India. Mol Biol Rep 48, 5587–5605 (2021). https://doi.org/10.1007/s11033-021-06355-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06355-4