Abstract
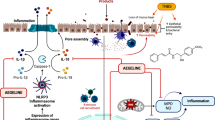
Chronic stress is linked to liver injury by increasing intestinal permeability to lipopolysaccharide (LPS), which in turn can result in systemic and liver inflammation and damage. Beneficial effect of honey in the prevention of liver injury has been shown in previous studies, but mechanisms underlying are still less known. Here, we examined the therapeutic impacts of honey on intestinal nuclear factor-κB (NF-κB; an important regulator of stress-induced immune and inflammatory responses) and ileal tight junction (TJ) proteins of claudin-1 and ZO-1, serum LPS, liver inflammation and oxidative markers of malondialdehyde (MDA), nitric oxide (NO), (erythroid-derived 2)-like 2 (Nrf2), tumor necrosis factor (TNF)-α and total antioxidant capacity (TAC) following chronic unpredictable mild stress (CUMS) using Western blotting, ELISA kit and spectrophotometry. Male rats were subjected to CUMS for 28 consecutive days. Honey (0.2 and 2 g/kg/day, by gavage) was administered pretreatment (10 days) and during stress. Honey reduced stress-induced LPS elevation by preventing reduction in the intestinal TJ proteins of claudin-1 and ZO-1, while did not affect NF-kB levels. In liver, honey significantly suppressed stress-induced increase in MDA, NO, TNF-α and Nrf2 expression and normalized TAC. Noteworthy, honey high-dose provoked a greater decrease in TNF-α, Nrf2 and LPS levels than honey low-dose. Together, our study indicated that honey protects against stress-induced liver damage by modulating at least two pathways; intestinal barrier protection via increased TJ protein complex expression, and hepatic TAC protection that may be involved in the inhibition of MDA, NO, TNF-α and Nrf2 expression.






Similar content being viewed by others
Change history
24 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11033-021-06296-y
References
Joung JY, Cho JH, Kim YH, et al (2019) A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 9
Roehlen N, Suarez AAR, El Saghire H et al (2020) Tight junction proteins and the biology of hepatobiliary disease. Int. J. Mol, Sci, p 21
Meddings JB, Swain MG (2000) Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 119:1019–1028. https://doi.org/10.1053/gast.2000.18152
Rodiño-Janeiro BK, Alonso-Cotoner C, Pigrau M et al (2015) Role of corticotropin-releasing factor in gastrointestinal permeability. J Neurogastroenterol Motil 21:33–50
Bhattarai Y (2018) Microbiota-gut-brain axis: interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil. https://doi.org/10.1111/nmo.13366
Fukui H (2015) Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol 7:425–442. https://doi.org/10.4254/wjh.v7.i3.425
Zheng G, Victor Fon G, Meixner W et al (2017) Chronic stress and intestinal barrier dysfunction: glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci Rep. https://doi.org/10.1038/s41598-017-04755-w
Demaude J, Salvador-Cartier C, Fioramonti J et al (2006) Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut 55:655–661. https://doi.org/10.1136/gut.2005.078675
Nicoletti A, Ponziani FR, Biolato M et al (2019) Intestinal permeability in the pathogenesis of liver damage: from non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol 25:4814–4834
Nguyen-Lefebvre AT, Horuzsko A (2015) Kupffer Cell Metabolism and Function. J Enzymol Metab 1:
Adachi Y, Moore LE, Bradford BU et al (1995) Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108:218–224. https://doi.org/10.1016/0016-5085(95)90027-6
Choi Y, Abdelmegeed MA, Song BJ (2018) Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J Nutr Biochem 55:12–25. https://doi.org/10.1016/j.jnutbio.2017.11.011
Rubio-Ruiz ME, Guarner-Lans V, Cano-Martínez A et al (2019) Resveratrol and quercetin administration improves antioxidant DEFENSES and reduces fatty liver in metabolic syndrome rats. Molecules. https://doi.org/10.3390/molecules24071297
Lambert JC, Zhou Z, Wang L et al (2003) Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther 305:880–886. https://doi.org/10.1124/jpet.102.047852
Dahiru D, Obidoa O (2008) Evaluation of the antioxidant effects of Ziziphus mauritiana lam. leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. African J Tradit Complement Altern Med 5:39. https://doi.org/10.4314/ajtcam.v5i1.31254
Bourogaa E, Nciri R, Mezghani-Jarraya R, -Sultan CR, Damak M, El Feki A et al (2012) Antioxidant activity and hepatoprotective potential of Hammada scoparia against ethanol-induced liver injury in rats. J Physiol Biochem 69:227–237. https://doi.org/10.1007/S13105-012-0206-7
Samarghandian S, Farkhondeh T, Samini F (2017) Honey and health: A review of recent clinical research. Pharmacognosy Res. 9:121–127
Martinotti S, Ranzato E (2018) Honey, wound repair and regenerative medicine. J. Funct, Biomater, p 9
Martinotti SRE (2014) Cellular and Molecular Mechanisms of Honey Wound Healing. Nova Publishers Inc, Hauppauge, NY, USA
Malkoç M, Yaman SÖ, Imamoğlu Y et al (2020) Anti-inflammatory, antioxidant and wound-healing effects of mad honey in streptozotocin-induced diabetic rats. J Apic Res 59:426–436. https://doi.org/10.1080/00218839.2019.1689036
Oryan A, Alemzadeh E, Moshiri A (2016) Biological properties and therapeutic activities of honey in wound healing: a narrative review and meta-analysis. J Tissue Viability 25:98–118. https://doi.org/10.1016/j.jtv.2015.12.002
Cianciosi D, Forbes-Hernández TY, Afrin S, et al (2018) Phenolic compounds in honey and their associated health benefits: a review. Molecules 23
Talebi M, Talebi M, Farkhondeh T, Samarghandian S (2020) Molecular mechanism-based therapeutic properties of hmechanism-based therapeutic properties of honey. Biomed Pharmacother 130:110590
Moloudian H, Abbasian S, Nassiri-Koopaei N, et al (2018) Characterization and classification of Iranian honey based on physicochemical properties and antioxidant activities, with chemometrics approach. Iran J Pharm Res 17:708–725. https://doi.org/https://doi.org/10.22037/ijpr.2018.2226
Devasvaran K, Tan JJ, Ng CT et al (2019) Malaysian tualang honey inhibits hydrogen peroxide-induced endothelial hyperpermeability. Oxid Med Cell Longev 2019:1202676. https://doi.org/10.1155/2019/1202676
Ali AM, Kunugi H (2019) Bee honey protects astrocytes against oxidative stress: a preliminary in vitro investigation. Neuropsychopharmac Rep 39:312–314. https://doi.org/10.1002/npr2.12079
Majtan J, Kumar P, Majtan T et al (2009) Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol 19:e73–e79. https://doi.org/10.1111/j.1600-0625.2009.00994.x
Wei L, Li Y, Tang W et al (2019) Chronic unpredictable mild stress in rats induces colonic inflammation. Front Physiol. https://doi.org/10.3389/fphys.2019.01228
Liu D, Wang Z, Gao Z et al (2014) Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res 271:116–121. https://doi.org/10.1016/j.bbr.2014.05.068
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9:799–809
Groschwitz KR, Hogan SP (2009) Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124:3–20
Salim SY, Söderholm JD (2011) Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis 17:362–381
Zong Y, Zhu S, Zhang S et al (2019) Chronic stress and intestinal permeability: lubiprostone regulates glucocorticoid receptor-mediated changes in colon epithelial tight junction proteins, barrier function, and visceral pain in the rodent and human. Neurogastroenterol Motil 31:e13477. https://doi.org/10.1111/nmo.13477
Oguz S, Salt O, Ibis AC et al (2018) Combined effectiveness of honey and immunonutrition on bacterial translocation secondary to obstructive jaundice in rats: experimental study. Med Sci Monit 24: 3374–3381. https://doi.org/https://doi.org/10.12659/MSM.907977
Gencay C, Kilicoglu SS, Kismet K et al (2008) Effect of honey on bacterial translocation and intestinal morphology in obstructive jaundice. World J Gastroenterol 14:3410–3415. https://doi.org/10.3748/wjg.14.3410
Maruhashi R, Eguchi H, Akizuki R et al (2019) Chrysin enhances anticancer drug-induced toxicity mediated by the reduction of claudin-1 and 11 expression in a spheroid culture model of lung squamous cell carcinoma cells. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-50276-z
Ranneh Y, Akim AM, Hamid HA et al (2019) Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr Metab 161(16):1–17. https://doi.org/10.1186/S12986-019-0341-Z
Aladaileh SH, Abukhalil MH, Saghir SAM et al (2019) Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules. https://doi.org/10.3390/biom9080346
Yaman T, Yener Z, Celik I (2016) Histopathological and biochemical investigations of protective role of honey in rats with experimental aflatoxicosis. BMC Complement Altern Med. https://doi.org/10.1186/s12906-016-1217-7
Kassim M, Mansor M, Al-Abd N, Yusoff KM (2012) Gelam honey has a protective effect against lipopolysaccharide (LPS)-induced organ failure. Int J Mol Sci 13:6370–6381. https://doi.org/10.3390/ijms13056370
Wang Z, Dou X, Li S et al (2014) Nuclear factor (erythroid-derived 2)-like 2 activation-induced hepatic very-low-density lipoprotein receptor overexpression in response to oxidative stress contributes to alcoholic liver disease in mice. Hepatology 59:1381–1392. https://doi.org/10.1002/hep.26912
Xie YL, Chu JG, Jian XM et al (2017) Curcumin attenuates lipopolysaccharide/D-galactosamine-induced acute liver injury by activating Nrf2 nuclear translocation and inhibiting NF-kB activation. Biomed Pharmacother 91:70–77. https://doi.org/10.1016/j.biopha.2017.04.070
Tian Y, Li Z, Shen B et al (2017) Protective effects of morin on lipopolysaccharide/D-galactosamine-induced acute liver injury by inhibiting TLR4/NF-κB and activating Nrf2/HO-1 signaling pathways. Int Immunopharmacol 45:148–155. https://doi.org/10.1016/j.intimp.2017.02.010
Peng X, Dai C, Liu Q et al (2018) Curcumin attenuates on carbon tetrachloride-induced acute liver injury in mice via modulation of the Nrf2/HO-1 and TGF-β1/Smad3 pathway. Molecules. https://doi.org/10.3390/molecules23010215
Xu D, Xu M, Jeong S et al (2019) The role of Nrf2 in liver disease: novel molecular mechanisms and therapeutic approaches. Front Pharmacol. https://doi.org/10.3389/fphar.2018.01428
Wullaert A, Bonnet MC, Pasparakis M (2011) NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res 21:146–158
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2
Ranneh Y, Ali F, Akim AM et al (2017) Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: a review. Appl Biol Chem 60:327–338
Raynaud A, Ghezali L, Gloaguen V et al (2013) Honey-induced macrophage stimulation: AP-1 and NF-κB activation and cytokine production are unrelated to LPS content of honey. Int Immunopharmacol 17:874–879. https://doi.org/10.1016/j.intimp.2013.09.014
Payne CM, Weber C, Crowley-Skillicorn C et al (2007) Deoxycholate induces mitochondrial oxidative stress and activates NF-κB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis 28:215–222. https://doi.org/10.1093/carcin/bgl139
Cho YE, Kim DK, Seo W et al (2019) Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450–2E1–mediated oxidative and nitrative stress. Hepatology. https://doi.org/10.1002/hep.30652
Acknowledgements
This work was supported by the Isfahan University of Medical Sciences (Grant number 198158 to M.G.). This work did involve animals (rats). The experimental procedures were approved by the Ethics Committee of the Isfahan University of Medical Sciences in accordance with ethical guidelines for the Care and Use of Laboratory Animals (IR.MUI.RESEARCH.REC.1398.533).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The correct spelling of the 3rd author’s name is Asiye Rafiee Sardooi.
Rights and permissions
About this article
Cite this article
Mehranfard, N., Yazdi, A., Sardooi, A.R. et al. Honey protects against chronic unpredictable mild stress induced- intestinal barrier disintegration and hepatic inflammation. Mol Biol Rep 47, 8475–8484 (2020). https://doi.org/10.1007/s11033-020-05888-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05888-4