Abstract
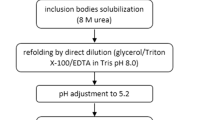
Recombinant form of granulocyte colony stimulating factor (G-CSF) was first approved by FDA in 1998 for chemotherapy induced neutropenia. However, despite production of its biosimilars, less expensive production of G-CSF could reduce the overall therapeutic cost. The aim of this study was to evaluate the possibility of producing biologically active recombinant G-CSF via a single step purification procedure mediated by a self-cleavable intein. G-CSF was expressed by E. coli BL21 (DE3) through IPTG induction, followed by its purification using pH optimization on a chitin column. Western blotting, ELISA, size exclusion chromatography, circular diachorism, peptide mapping, and in vitro assays were performed to compare the structural similarity and biological activity of the purified G-CSF with Neupogen™. Protein purification was confirmed by revealing a band of approximately 18.8 kDa on SDS-PAGE. Bioactivity and physicochemical assays based on the US pharmacopeia showed almost identical or acceptable ranges of similarities between recombinant G-CSF and Neopogen™. this study, biologically active soluble recombinant G-CSF was successfully produced with high purity without using chaotropic solvents through a one-step procedure. This shorter and more efficient purification procedure can reduce the cost and time of G-CSF production which makes its industrial production more cost-effective and might be also applicable for production of other biopharmaceuticals.





Similar content being viewed by others
References
Basu S, Dunn A, Ward A (2002) G-CSF: function and modes of action (review). Int JMol Med 10:3–10
Roberts AW (2005) G-CSF: a key regulator of neutrophil production, but that's not all. Growth Factors J 23:33–41
Kopf B, De Giorgi U, Vertogen B, Monti G, Molinari A, Turci D et al (2015) A randomized study comparing filgrastim versus lenograstim versus molgramostim plus chemotherapy for peripheral blood progenitor cell mobilization. Bone Marrow Transplant 38(6):407–412
Nahon S, Rastkhah M, Abdelghani MB et al (2016) Zarzio®, biosimilar of filgrastim, in prophylaxis of chemotherapy-induced neutropenia in routine practice: a French prospective multicentric study. Support Care Cancer 24(5):1991–1998
Davio K (2018) Pfizer launches biosimilar filgrastim, nivestym, at a substantial discount. https://www.centerforbiosimilars.com/news/pfizer-launches-biosimilar-filgrastim-nivestym-at-a-substantial-discount. Accessed 22 Dec 2019
Rad HS, Mousavi SL, Rasooli I, Amani J, Nadooshan MR (2013) EspA-Intimin chimeric protein, acandidate vaccine against Escherichia coli O157:H7. Iran J Microbiol 5:244–251
Miri A, Salimian J, Rezai E, Olad G, Sadati M, Arefpour-Tehrani MA et al (2013) Evaluation and comparison of immunization level between recombinant proteins of binding subunit of entrotoxigenic Escherichia coli and botulinum toxin. J Shahrekord Univ Med Sci 15:159–166
Rodríguez V, Lascani J, Asenjo JA, Andrews BA (2015) Production of cell-penetrating peptides in Escherichia coli using an intein-mediated system. Appl Biochem Biotechnol 175:3025–3037
Shafiee F, Minaiyan G, Moazen F, Jahanian-Najafabadi A (2017) Recombinant production and intein-mediated purification of an antimicrobial peptide, BR2. Int J Pep Res Ther 23:501–507
Ta DT, Redeker ES, Billen B, Reekmans G, Sikulu J, Noben JP et al (2015) An efficient protocol towards site-specifically clickable nanobodies in high yield: cytoplasmic expression in Escherichia coli combined with intein-mediated protein ligation. Protein Eng Des Sel 28(10):351–363
Perler FB (2002) InBase: The intein database. Nucleic Acids Res 30(1):383–384
Xu MQ, Evans TC Jr (2003) Purification of recombinant proteins from E. coli by engineered inteins. Methods Mol Biol 205:43–68
Zhao Z, Lu W, Dun B, Jin D, Ping S, Zhang W et al (2008) Purification of green fluorescent protein using a two-intein system. Appl Microbiol Biotechnol 77:1175–1180
Mathys S, Evans TC, Chute IC, Wu H, Chong S, Benner J et al (1999) Characterization of self-splicing mini-intein and its conversion into autocatalytic N- and C-terminal cleavage elements: facile production of protein building blocks for protein ligation. Gene 231:1–13
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Savar NS, Jahanian-Najafabadi A, Mahdavi M, Shokrgozar MA, Jafari A, Bouzari S (2014) In silico and in vivo studies of truncated forms of flagellin (FliC) of entero-aggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J Biotechnol 10:31–37
United States Pharmacopeia and National Formulary (USP 38-NF 33) (2016) United States Pharmacopeial Convention. Rockville, MD, US. https://online.uspnf.com/uspnf/document/GUID-AC788D41-90A2-4F36-A6E7-769954A9ED09_1_en-US. Accessed 18 Jan 2019
Bich HD, Han-Bong R, Phuong H, Bon-Kyung K, Han C (2014) Soluble prokaryotic overexpression and purification of bioactive human granulocyte colony-stimulating factor by maltose binding protein and protein disulfide isomerase. PLoS ONE 9:e89906
Pezza J, Allen K, Tolan R (2004) Intein-mediated purification of a recombinantly expressed peptide. Chem Comm 21:2412–2413
Srinivsa Babu K, Muthukumaran T, Antony A, Prem Singh Samuel SD, Balamurali M, Murugan V et al (2009) Single step intein-mediated purification of hGMCSF expressed in salt-inducible E. coli. Biotechnol Lett 31:659–664
Lahiry A, Fan Y, Stimple SD, Raith M, Wood DW (2018) Inteins as tools for tagless and traceless protein purification. J Chem Technol Biotechnol 93:1827–1835
Sun Z, Chen J, Yao H, Liu L, Wang J, Zhang J et al (2005) Use of SspdnaB derivedd mini-intein as a fusion partner for production of recombinant human brain natriuretic peptide in Escherichia coli. Prot Exp Pur 43:26–32
Humphries HE, Christodoulides M, Heckels JE (2002) Expression of the class 1 outer-membrane protein of Neisseria meningitidis in Escherichia coli and purification using self-cleavable affinity tag. Prot Exp Pur 26:243–248
Bae C, Yang D, Lee J, Park Y-H (1999) Improved process for production of recombinant yeast-derived monomeric human G-CSF. Appl Microbial Biotechnol 52:338–344
Gill RT, Cha HJ, Jain A, Rao G, Bentley WE (1998) Generating controlled reducing environments in aerobic recombinant Escherichia coli fermentations: effects on cell growth, oxygen uptake, heat shock protein expression, and in vivo CAT activity. Biotechnol Bioeng 59:248–255
Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC et al (1986) Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloidcells. Science 232:61–65
Herman AC et al (1996) Characterization, formulation, and stability of Neupogen (Filgrastim), a recombinant human granulocyte-colony stimulating factor. Pharm Biotechnol. 9:303–328
Aapro M, Bohlius J, Cameron D, Dal Lago L, Donnelly JP, Kearney N et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Can 47:8–32
Heyland J, Blank LM, Schmid A (2011) Quantification of metabolic limitations during recombinant protein production in Escherichia coli. J Biotechnol 155:178–180
Khow O, Suntrarachun S (2012) Strategies for production of active eukaryotic proteins in bacterial expression system. Asi Pac J Trop Biomed 2:159–162
Zhao Y, He W, Liu W-F, Liu C-C, Feng L-K, Sun L et al (2012) Two distinct states of Escherichia coli cells that overexpress recombinant heterogeneous β-galactosidase. J Biol Chem 287:9259–9266
Gascon P (2012) Presently available biosimilars in hematology-oncology: G-CSF. Target Oncol 7:29–34
Acknowledgements
The authors are grateful to thank Ms. Fatemeh Moazen for her technical assistance. This work has been financially supported by the Research Deputy of Isfahan University of Medical Sciences via Grants Nos. 394698 and 195210.
Author information
Authors and Affiliations
Contributions
AJN and FS designed the study. SS performed the experiments. SS, FS and AJN analysed the results. SS and FS prepared first draft of the manuscript. AJN finalized and submitted the manuscript. AJN prepared the revised manuscript according to the reviewers comments and submitted the final revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2020_5404_MOESM1_ESM.jpg
Supplementary material 1 Supplementary Figure 1. G-CSF (Filgrastim) protein sequence retrieved from www.drugbank.ca (https://www.drugbank.ca/drugs/DB00099), in addition to some information on the protein characteristics. (JPEG 247 kb)
11033_2020_5404_MOESM2_ESM.jpg
Supplementary material 2 Supplementary Figure 2. Spectrum of the purified G-CSF following size-exclusion chromatography by FPLC. The purity of the recombinant G-CSF was confirmed by revealing a sharp signal in the spectrum (JPEG 359 kb)
Rights and permissions
About this article
Cite this article
Sima, S., Shafiee, F. & Jahanian-Najafabadi, A. Expression and one step intein-mediated purification of biologically active human G-CSF in Escherichia coli. Mol Biol Rep 47, 2861–2869 (2020). https://doi.org/10.1007/s11033-020-05404-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05404-8