Abstract
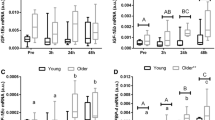
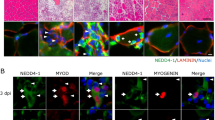
Skeletal muscle regeneration is mostly dependent on muscle satellite cells. Proper muscle regeneration requires enough number of satellite cells. Recent studies have suggested that the number of satellite cells in skeletal muscle declines as we age, leading to the impairment of muscle regeneration in older population. Our earlier study demonstrated that zinc finger transcription factor early growth response 3 (Egr3) plays an important role for maintaining the number of myoblasts, suggesting that age-related decrease in muscle satellite cell should be associated with the expression levels of Egr3. The aim of this study was to investigate whether aging would alter the Egr3 expression in satellite cells. A couple groups of male C57BL/6J mice were examined in this study: young (3 Mo) and old (17 Mo). Immunohistochemical staining showed that the satellite cell number decreased in normal and injured muscles of old mice. In fluorescence-activated cell sorting-isolated muscle satellite cells from normal and injured muscles, the mRNA expression of Egr3 was significantly decreased with age regardless of injury. In harmony with these results, Pax7 mRNA levels also decreased in the satellite cells from old mice. Alternatively, inhibition of Egr3 expression by shRNA decreased Pax7 protein expression in cultured myoblasts. These results suggest that Egr3 is associated with the age-related decline of muscle satellite cells in older population. Also, Egr3 might be implicated in the regulation of Pax7. Therefore, the loss of Egr3 expression may elucidate attenuated MSCs function and muscle regeneration in older age.







Similar content being viewed by others
References
Garcia-Prat L, Sousa-Victor P, Munoz-Canoves P (2013) Functional dysregulation of stem cells during aging: a focus on skeletal muscle stem cells. FEBS J 280(17):4051–4062. https://doi.org/10.1111/febs.12221
Hwang AB, Brack AS (2018) Muscle stem cells and aging. Curr Top Dev Biol 126:299–322. https://doi.org/10.1016/bs.ctdb.2017.08.008
Brack AS, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3(3):226–237. https://doi.org/10.1007/s12015-007-9000-2
Joanisse S, Nederveen JP, Baker JM, Snijders T, Iacono C, Parise G (2016) Exercise conditioning in old mice improves skeletal muscle regeneration. FASEB J 30(9):3256–3268. https://doi.org/10.1096/fj.201600143RR
Brack AS, Bildsoe H, Hughes SM (2005) Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118(Pt 20):4813–4821. https://doi.org/10.1242/jcs.02602
Shefer G, Rauner G, Stuelsatz P, Benayahu D, Yablonka-Reuveni Z (2013) Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice. FEBS J 280(17):4063–4073. https://doi.org/10.1111/febs.12228
Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z (2006) Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294(1):50–66. https://doi.org/10.1016/j.ydbio.2006.02.022
Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA (2007) A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25(4):885–894. https://doi.org/10.1634/stemcells.2006-0372
Day K, Shefer G, Shearer A, Yablonka-Reuveni Z (2010) The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol 340(2):330–343. https://doi.org/10.1016/j.ydbio.2010.01.006
Schultz E, Lipton BH (1982) Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev 20(4):377–383. https://doi.org/10.1016/0047-6374(82)90105-1
Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P (2014) Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506(7488):316–321. https://doi.org/10.1038/nature13013
Collins-Hooper H, Woolley TE, Dyson L, Patel A, Potter P, Baker RE, Gaffney EA, Maini PK, Dash PR, Patel K (2012) Age-related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells 30(6):1182–1195. https://doi.org/10.1002/stem.1088
Chakkalakal JV, Jones KM, Basson MA, Brack AS (2012) The aged niche disrupts muscle stem cell quiescence. Nature 490(7420):355–360. https://doi.org/10.1038/nature11438
Morioka S, Goto K, Kojima A, Naito T, Matsuba Y, Akema T, Fujiya H, Sugiura T, Ohira Y, Beppu M, Aoki H, Yoshioka T (2008) Functional overloading facilitates the regeneration of injured soleus muscles in mice. J Physiol Sci 58(6):397–404. https://doi.org/10.2170/physiolsci.RP004008
O’Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM (1999) The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci 22(4):167–173. https://doi.org/10.1016/S0166-2236(98)01343-5
Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD (2005) Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol 6(5):472–480. https://doi.org/10.1038/ni1193
Sumitomo S, Fujio K, Okamura T, Morita K, Ishigaki K, Suzukawa K, Kanaya K, Kondo K, Yamasoba T, Furukawa A, Kitahara N, Shoda H, Shibuya M, Okamoto A, Yamamoto K (2013) Transcription factor early growth response 3 is associated with the TGF-beta1 expression and the regulatory activity of CD4-positive T cells in vivo. J Immunol 191(5):2351–2359. https://doi.org/10.4049/jimmunol.1202106
Sumitomo S, Fujio K, Okamura T, Yamamoto K (2013) Egr2 and Egr3 are the unique regulators for systemic autoimmunity. Jak-Stat 2(2):e23952. https://doi.org/10.4161/jkst.23952
O’Donovan KJ, Wilkens EP, Baraban JM (1998) Sequential expression of Egr-1 and Egr-3 in hippocampal granule cells following electroconvulsive stimulation. J Neurochem 70(3):1241–1248. https://doi.org/10.1046/j.1471-4159.1998.70031241.x
Suehiro J, Hamakubo T, Kodama T, Aird WC, Minami T (2010) Vascular endothelial growth factor activation of endothelial cells is mediated by early growth response-3. Blood 115(12):2520–2532. https://doi.org/10.1182/blood-2009-07-233478
Oliveira Fernandes M, Tourtellotte WG (2015) Egr3-dependent muscle spindle stretch receptor intrafusal muscle fiber differentiation and fusimotor innervation homeostasis. J Neurosci 35(14):5566–5578. https://doi.org/10.1523/JNEUROSCI.0241-15.2015
Tourtellotte WG, Keller-Peck C, Milbrandt J, Kucera J (2001) The transcription factor Egr3 modulates sensory axon-myotube interactions during muscle spindle morphogenesis. Dev Biol 232(2):388–399. https://doi.org/10.1006/dbio.2001.0202
Kurosaka M, Ogura Y, Funabashi T, Akema T (2017) Early growth response 3 (Egr3) contributes a maintenance of C2C12 myoblast proliferation. J Cell Physiol 232(5):1114–1122. https://doi.org/10.1002/jcp.25574
Liu L, Cheung TH, Charville GW, Rando TA (2015) Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc 10(10):1612–1624. https://doi.org/10.1038/nprot.2015.110
Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka-Reuveni Z, Reyes M (2010) Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS ONE 5(6):e10920. https://doi.org/10.1371/journal.pone.0010920
Ogura Y, Mishra V, Hindi SM, Kuang S, Kumar A (2013) Proinflammatory cytokine tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) suppresses satellite cell self-renewal through inversely modulating Notch and NF-kappaB signaling pathways. J Biol Chem 288(49):35159–35169. https://doi.org/10.1074/jbc.M113.517300
Kitajima Y, Ono Y (2018) Visualization of PAX7 protein dynamics in muscle satellite cells in a YFP knock-in-mouse line. Skelet Muscle 8(1):26. https://doi.org/10.1186/s13395-018-0174-x
Hindi SM, Kumar A (2016) TRAF6 regulates satellite stem cell self-renewal and function during regenerative myogenesis. J Clin Investig 126(1):151–168. https://doi.org/10.1172/JCI81655
Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS (2003) Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278(10):8826–8836. https://doi.org/10.1074/jbc.M209879200
Ogura Y, Hindi SM, Sato S, Xiong G, Akira S, Kumar A (2015) TAK1 modulates satellite stem cell homeostasis and skeletal muscle repair. Nat Commun 6:10123. https://doi.org/10.1038/ncomms10123
Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS (2010) Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401(2):318–320. https://doi.org/10.1016/j.ab.2010.02.036
Kumar A, Takada Y, Boriek AM, Aggarwal BB (2004) Nuclear factor-kappaB: its role in health and disease. J Mol Med (Berl) 82(7):434–448. https://doi.org/10.1007/s00109-004-0555-y
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132(3):344–362. https://doi.org/10.1016/j.cell.2008.01.020
Fulle S, Sancilio S, Mancinelli R, Gatta V, Di Pietro R (2013) Dual role of the caspase enzymes in satellite cells from aged and young subjects. Cell Death Dis 4:e955. https://doi.org/10.1038/cddis.2013.472
Jejurikar SS, Henkelman EA, Cederna PS, Marcelo CL, Urbanchek MG, Kuzon WM Jr (2006) Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Exp Gerontol 41(9):828–836. https://doi.org/10.1016/j.exger.2006.06.053
Lepper C, Partridge TA, Fan CM (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138(17):3639–3646. https://doi.org/10.1242/dev.067595
Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S (2012) A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30(2):243–252. https://doi.org/10.1002/stem.775
von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA (2013) Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA 110(41):16474–16479. https://doi.org/10.1073/pnas.1307680110
Olguin HC, Yang Z, Tapscott SJ, Olwin BB (2007) Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol 177(5):769–779. https://doi.org/10.1083/jcb.200608122
Yin H, Price F, Rudnicki MA (2013) Satellite cells and the muscle stem cell niche. Physiol Rev 93(1):23–67. https://doi.org/10.1152/physrev.00043.2011
Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA (1996) MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10(10):1173–1183
Hernandez-Hernandez JM, Garcia-Gonzalez EG, Brun CE, Rudnicki MA (2017) The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin Cell Dev Biol 72:10–18. https://doi.org/10.1016/j.semcdb.2017.11.010
Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR (2004) Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166(3):347–357. https://doi.org/10.1083/jcb.200312007
Musaro A, Cusella De Angelis MG, Germani A, Ciccarelli C, Molinaro M, Zani BM (1995) Enhanced expression of myogenic regulatory genes in aging skeletal muscle. Exp Cell Res 221(1):241–248. https://doi.org/10.1006/excr.1995.1372
Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM (2003) MyoD and myogenin protein expression in skeletal muscles of senile rats. Cell Tissue Res 311(3):401–416. https://doi.org/10.1007/s00441-002-0686-9
Fuggle N, Shaw S, Dennison E, Cooper C (2017) Sarcopenia. Best Pract Res Clin Rheumatol 31(2):218–242. https://doi.org/10.1016/j.berh.2017.11.007
Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA (2015) Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21(1):76–80. https://doi.org/10.1038/nm.3710
Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, Kardon G (2015) Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun 6:7087. https://doi.org/10.1038/ncomms8087
Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortes-Lopez M, Tan A, Flaherty M, Miura P, Dirksen RT, Chakkalakal JV (2017) Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife. https://doi.org/10.7554/eLife.26464
Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA (2017) Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20(1):56–69. https://doi.org/10.1016/j.stem.2016.09.010
Verdijk LB, Dirks ML, Snijders T, Prompers JJ, Beelen M, Jonkers RA, Thijssen DH, Hopman MT, Van Loon LJ (2012) Reduced satellite cell numbers with spinal cord injury and aging in humans. Med Sci Sports Exerc 44(12):2322–2330. https://doi.org/10.1249/MSS.0b013e3182667c2e
Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ (2007) Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292(1):E151–E157. https://doi.org/10.1152/ajpendo.00278.2006
Acknowledgements
Ms. Chiaki Kakehashi is acknowledged for animal maintenance. We thank Dr. James Clemons for his proofreading and statistical consultation of this manuscript. This work was partially supported by a research grant from the General Insurance Association of Japan to Y.O., by the Public Health Research Foundation to Y.O. and by JSPS KAKENHI Grants Number 18K10835 to Y.O., 15K01633 to M.K., 15K01632 to H.F.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogura, Y., Sato, S., Kurosaka, M. et al. Age-related decrease in muscle satellite cells is accompanied with diminished expression of early growth response 3 in mice. Mol Biol Rep 47, 977–986 (2020). https://doi.org/10.1007/s11033-019-05189-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05189-5