Abstract
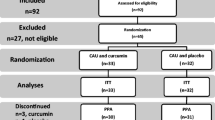
Bipolar disorder (BPD) is a severe and chronic mental disease with high rates of social and functional disability. To explain the emergence and maintenance of BPD, increasing attention has been focused on dimensions of inflammation and oxidative stress (OTS). Coenzyme Q10 (CoQ10) is known for its anti-oxidant and anti-inflammatory effects; accordingly, the aim of the present study was to investigate, if compared to placebo, adjuvant CoQ10 might favorably impact on serum levels of inflammatory and OTS biomarkers in patients with BPD during their depressive phase. A total of 89 BPD patients, currently in a depressive episode were allocated by block randomization either to the adjuvant CoQ10 (200 mg/day) condition or to the placebo condition. At baseline and 8 weeks later at the end of the study, serum levels of total antioxidant capacity (TAC), total thiol groups (TTG), catalase activity (CAT), nitric oxide (NO), malondialdehyde (MDA), tumor necrosis factor-alpha (TNF-α), interlukin-6 (IL-6), and IL-10 were assessed. 69 patients completed the 8-week lasting study. Compared to baseline and to the placebo condition, serum levels of TTG and TAC significantly increased, and TNF-α, IL-10, and NO statistically decreased over time in the adjuvant CoQ10 condition. No statistically significant changes were observed for CAT, MDA, and IL-6. The pattern of results suggests that compared to placebo and over a time lapse of 8 weeks, adjuvant CoQ10 favorably impacted on OTS and inflammatory biomarkers in patients with BPD during the depressive episode. Thus, CoQ10 might be considered a safe and effective strategy for treatment of patients with BPD during their depressive phase.

Similar content being viewed by others
References
Fagiolini A, Forgione R, Maccari M, Cuomo A, Morana B, Dell’Osso MC, Pellegrini F, Rossi A (2013) Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord 148(2):161–169
Sanchez-Moreno J, Martinez-Aran A, Tabarés-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos J (2009) Functioning and disability in bipolar disorder: an extensive review. Psychother Psychosom 78(5):285–297
Kupfer DJ (2005) The increasing medical burden in bipolar disorder. JAMA 293(20):2528–2530
Tondo L, Isacsson G, Baldessarini RJ (2003) Suicidal behaviour in bipolar disorder. CNS Drugs 17(7):491–511
Berk M, Berk L, Davey CG, Moylan S, Giorlando F, Singh AB, Kalra H, Dodd S, Malhi GS (2013) Treatment of bipolar depression. Med J Aust 199(6 Suppl):S32–S35
Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS, Zarate CA (2003) The underlying neurobiology of bipolar disorder. World Psychiatry 2(3):136
Maletic V, Raison C (2014) Integrated neurobiology of bipolar disorder. Front Psychiatry 5:98
Munkholm K, Brauner JV, Kessing LV, Vinberg M (2013) Cytokines in bipolar disorder vs healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res 47(9):1119–1133. https://doi.org/10.1016/j.jpsychires.2013.05.018
Rosenblat JD, Cha DS, Mansur RB, McIntyre RS (2014) Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 53:23–34
Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J (2011) Depression’s multiple comorbidities explained by (neuro) inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol Lett 32(1):7–24
Moylan S, Maes M, Wray N, Berk M (2013) The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18(5):595
Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH (2013) A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70(1):31–41
Ortiz-Domínguez A, Hernández ME, Berlanga C, Gutiérrez-Mora D, Moreno J, Heinze G, Pavón L (2007) Immune variations in bipolar disorder: phasic differences. Bipolar Disord 9(6):596–602
Modabbernia A, Taslimi S, Brietzke E, Ashrafi M (2013) Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry 74(1):15–25
Barbosa IG, Bauer ME, Machado-Vieira R, Teixeira AL (2014) Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast. https://doi.org/10.1155/2014/360481
Reynolds JL, Ignatowski TA, Sud R, Spengler RN (2004) Brain-derived tumor necrosis factor-alpha and its involvement in noradrenergic neuron functioning involved in the mechanism of action of an antidepressant. J Pharmacol Exp Ther 310(3):1216–1225. https://doi.org/10.1124/jpet.104.067835
Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PV, Amminger P, McGorry P, Malhi GS (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35(3):804–817. https://doi.org/10.1016/j.neubiorev.2010.10.001
Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, Yatham LN (2008) Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord 111(2–3):135–144. https://doi.org/10.1016/j.jad.2008.04.013
Rahman T, Hosen I, Islam MT, Shekhar HU (2012) Oxidative stress and human health. Adv Biosci Biotechnol 3(07):997
Kato T, Iwamoto K, Kakiuchi C, Kuratomi G, Okazaki Y (2005) Genetic or epigenetic difference causing discordance between monozygotic twins as a clue to molecular basis of mental disorders. Mol Psychiatry 10(7):622–630. https://doi.org/10.1038/sj.mp.4001662
Brown NC, Andreazza AC, Young LT (2014) An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res 218(1):61–68
Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Bunney WE, Vawter MP (2008) Mitochondrial involvement in psychiatric disorders. Ann Med 40(4):281–295. https://doi.org/10.1080/07853890801923753
Maes M, Galecki P, Chang YS, Berk M (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 35(3):676–692
Kim YK, Jung HG, Myint AM, Kim H, Park SH (2007) Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord 104(1–3):91–95. https://doi.org/10.1016/j.jad.2007.02.018
McNamara RK, Jandacek R, Rider T, Tso P (2011) Chronic risperidone normalizes elevated pro-inflammatory cytokine and C-reactive protein production in omega-3 fatty acid deficient rats. Eur J Pharmacol 652(1–3):152–156. https://doi.org/10.1016/j.ejphar.2010.11.010
Berger I, Segal I, Shmueli D, Saada A (2010) The effect of antiepileptic drugs on mitochondrial activity: a pilot study. J Child Neurol 25(5):541–545. https://doi.org/10.1177/0883073809352888
Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F (2014) Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS ONE 9(5):e96905
Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI (2008) N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry 64(6):468–475
Forester BP, Harper DG, Georgakas J, Ravichandran C, Madurai N, Cohen BM (2015) Antidepressant effects of open label treatment with Coenzyme Q10 in geriatric bipolar depression. J Clin Psychopharmacol 35(3):338
Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20(6):591–598
Pandya CD, Howell KR, Pillai A (2013) Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 46:214–223
Elbaky NAA, El-Orabi NF, Fadda LM, Abd-Elkader OH, Ali HM (2018) Role of N-acetylcysteine and coenzyme Q10 in the amelioration of myocardial energy expenditure and oxidative stress, induced by carbon tetrachloride intoxication in rats. Dose-Response 16(3):1559325818790158. https://doi.org/10.1177/1559325818790158
Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Jazayeri S, Reza Gohari M (2013) Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int J Neurosci 123(11):776–782. https://doi.org/10.3109/00207454.2013.801844
Mehrpooya M, Yasrebifar F, Haghighi M, Mohammadi Y, Jahangard L (2018) Evaluating the effect of coenzyme Q10 augmentation on treatment of bipolar depression: a double-blind controlled clinical trial. J Clin Psychopharmacol 38(5):460–466. https://doi.org/10.1097/jcp.0000000000000938
Association AP (2013) Diagnostic and statistical manual of mental disorders. BMC Med 17:133–137
Ahmadpanah M, Sheikhbabaei M, Haghighi M, Roham F, Jahangard L, Akhondi A, Sadeghi Bahmani D, Bajoghli H, Holsboer-Trachsler E, Brand S (2016) Validity and test-retest reliability of the Persian version of the Montgomery-Asberg depression rating scale. Neuropsychiatr Dis Treat 12:603–607. https://doi.org/10.2147/ndt.s103869
Benzie IF, Szeto YT (1999) Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem 47(2):633–636
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
McNamara RK, Lotrich FE (2012) Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert Rev Neurother 12(9):1143–1161. https://doi.org/10.1586/ern.12.98
Doganavsargil-Baysal O, Cinemre B, Aksoy UM, Akbas H, Metin O, Fettahoglu C, Gokmen Z, Davran F (2013) Levels of TNF-alpha, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Hum Psychopharmacol 28(2):160–167. https://doi.org/10.1002/hup.2301
Brietzke E, Stertz L, Fernandes BS, Kauer-Sant’anna M, Mascarenhas M, Escosteguy Vargas A, Chies JA, Kapczinski F (2009) Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord 116(3):214–217. https://doi.org/10.1016/j.jad.2008.12.001
Cunha AB, Andreazza AC, Gomes FA, Frey BN, da Silveira LE, Goncalves CA, Kapczinski F (2008) Investigation of serum high-sensitive C-reactive protein levels across all mood states in bipolar disorder. Eur Arch Psychiatry Clin Neurosci 258(5):300–304. https://doi.org/10.1007/s00406-007-0797-0
Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L (2009) Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiat 66(3):287–292. https://doi.org/10.1016/j.biopsych.2009.01.030
Castaño-Ramírez OM, Sepúlveda-Arias JC, Duica K, Zuluaga AMD, Vargas C, López-Jaramillo C (2018) Inflammatory markers in the staging of bipolar disorder: a systematic review of the literature. Revista Colombiana de Psiquiatría (English ed) 47(2):119–128
Kunz M, Cereser KM, Goi PD, Fries GR, Teixeira AL, Fernandes BS, Belmonte-de-Abreu PS, Kauer-Sant’Anna M, Kapczinski F, Gama CS (2011) Serum levels of IL-6, IL-10 and TNF-alpha in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999) 33(3):268–274
Franchini L, Zanardi R, Smeraldi E, Gasperini M (1999) Early onset of lithium prophylaxis as a predictor of good long-term outcome. Eur Arch Psychiatry Clin Neurosci 249(5):227–230
Chatterjee S (2016) Oxidative stress, inflammation, and disease. Oxidative Stress and Biomaterials. Elsevier, Amsterdam, pp 35–58
Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, Jope RS (2010) Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology 35(8):1761–1774. https://doi.org/10.1038/npp.2010.43
Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, Bowden CL, Soares JC (2008) Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol 23(2):87–94. https://doi.org/10.1002/hup.912
Stolk P, Souverein PC, Wilting I, Leufkens HG, Klein DF, Rapoport SI, Heerdink ER (2010) Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins Leukot Essent Fatty Acids 82(1):9–14. https://doi.org/10.1016/j.plefa.2009.10.007
Sarris J, Mischoulon D, Schweitzer I (2011) Adjunctive nutraceuticals with standard pharmacotherapies in bipolar disorder: a systematic review of clinical trials. Bipolar Disord 13(5–6):454–465. https://doi.org/10.1111/j.1399-5618.2011.00945.x
Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Doring F (2008) Functions of coenzyme Q10 in inflammation and gene expression. BioFactors (Oxford, England) 32(1–4):179–183
Tarry-Adkins JL, Fernandez-Twinn DS, Hargreaves IP, Neergheen V, Aiken CE, Martin-Gronert MS, McConnell JM, Ozanne SE (2015) Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am J Clin Nutr 103(2):579–588
Wang XL, Rainwater DL, Mahaney MC, Stocker R (2004) Cosupplementation with vitamin E and coenzyme Q10 reduces circulating markers of inflammation in baboons. Am J Clin Nutr 80(3):649–655
Fan L, Feng Y, Chen G-C, Qin L-Q, Fu C-l, Chen L-H (2017) Effects of coenzyme Q10 supplementation on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 119:128–136
Stocker R, Bowry VW, Frei B (1991) Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci USA 88(5):1646–1650
Landi L, Cabrini L, Fiorentini D, Stefanelli C, Pedulli GF (1992) The antioxidant activity of ubiquinol-3 in homogeneous solution and in liposomes. Chem Phys Lipid 61(2):121–130
Kagan V, Serbinova E, Packer L (1990) Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun 169(3):851–857
Rivara MB, Yeung CK, Robinson-Cohen C, Phillips BR, Ruzinski J, Rock D, Linke L, Shen DD, Ikizler TA, Himmelfarb J (2017) Effect of coenzyme Q10 on biomarkers of oxidative stress and cardiac function in hemodialysis patients: the CoQ10 biomarker trial. Am J Kidney Dis 69(3):389–399. https://doi.org/10.1053/j.ajkd.2016.08.041
Sakata T, Furuya R, Shimazu T, Odamaki M, Ohkawa S, Kumagai H (2008) Coenzyme Q10 administration suppresses both oxidative and antioxidative markers in hemodialysis patients. Blood Purif 26(4):371–378. https://doi.org/10.1159/000135605
Lee B-J, Tseng Y-F, Yen C-H, Lin P-T (2013) Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: a randomized, placebo-controlled trial. Nutr J 12(1):142
Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, Littarru GP (2007) Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J 28(18):2249–2255
Abou-Raya A, Abou-Raya S, Helmii M (2014) THU0305 effect of oral coenzyme Q10 supplementation on clinical symptoms and oxidative stress in fibromyalgia patients: a randomized trial. Ann Rheum Dis 73(Suppl 2):288–289. https://doi.org/10.1136/annrheumdis-2014-eular.2611
Jorat MV, Tabrizi R, Kolahdooz F, Akbari M, Salami M, Heydari ST, Asemi Z (2019) The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 27(2):233–248
Lee BJ, Lin YC, Huang YC, Ko YW, Hsia S, Lin PT (2012) The relationship between coenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronary artery disease. Sci World J 2012:792756. https://doi.org/10.1100/2012/792756
Kedziora-Kornatowska K, Czuczejko J, Motyl J, Szewczyk-Golec K, Kozakiewicz M, Pawluk H, Kedziora J, Blaszczak R, Banach M, Rysz J (2010) Effects of coenzyme Q10 supplementation on activities of selected antioxidative enzymes and lipid peroxidation in hypertensive patients treated with indapamide. A pilot study. Arch Med Sci AMS 6(4):513–518. https://doi.org/10.5114/aoms.2010.14461
Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF (2009) Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J Neurochem 109(5):1427–1439. https://doi.org/10.1111/j.1471-4159.2009.06074.x
Wadsworth TL, Bishop JA, Pappu AS, Woltjer RL, Quinn JF (2008) Evaluation of coenzyme Q as an antioxidant strategy for Alzheimer’s disease. J Alzheimer’s Dis 14(2):225–234
Gvozdjakova A, Kucharska J, Ostatnikova D, Babinska K, Nakladal D, Crane FL (2014) Ubiquinol improves symptoms in children with autism. Oxid Med Cell Longev 2014:798957. https://doi.org/10.1155/2014/798957
Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E (2009) Lower plasma Coenzyme Q 10 in depression: a marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuroendocrinol Lett 30(4):462–469
Forester BP, Zuo CS, Ravichandran C, Harper DG, Du F, Kim S, Cohen BM, Renshaw PF (2012) Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. J Geriatr Psychiatry Neurol 25(1):43–50. https://doi.org/10.1177/0891988712436688
Aboul-Fotouh S (2013) Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol Biochem Behav 104:105–112. https://doi.org/10.1016/j.pbb.2012.12.027
Kagal UA, Angadi NB (2017) Effect of coenzyme Q10 on depression paradigms in male Wistar rats and Swiss albino mice. Natl J Physiol Pharm Pharmacol 7(8):802
Ferrante KL, Shefner J, Zhang H, Betensky R, O’Brien M, Yu H, Fantasia M, Taft J, Beal MF, Traynor B, Newhall K, Donofrio P, Caress J, Ashburn C, Freiberg B, O’Neill C, Paladenech C, Walker T, Pestronk A, Abrams B, Florence J, Renna R, Schierbecker J, Malkus B, Cudkowicz M (2005) Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology 65(11):1834–1836. https://doi.org/10.1212/01.wnl.0000187070.35365.d7
Bonakdar RA, Guarneri E (2005) Coenzyme Q10. Am Fam Physician 72(6):1065–1070
Acknowledgement
This study was supported by Vice-Chancellor of Research and Technology of Hamadan University of Medical Sciences, Hamadan, Iran (No: 9605032798). The authors thank all patients for helping and participating in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jahangard, L., Yasrebifar, F., Haghighi, M. et al. Influence of adjuvant Coenzyme Q10 on inflammatory and oxidative stress biomarkers in patients with bipolar disorders during the depressive episode. Mol Biol Rep 46, 5333–5343 (2019). https://doi.org/10.1007/s11033-019-04989-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04989-z