Abstract
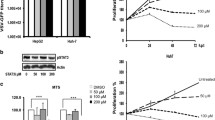
Colorectal cancer (CRC) is the third most common cancer in both men and women. Oncolytic viral-based therapy methods seem to be promising for CRC treatment. Vesicular stomatitis virus (VSV) is considered as a potent candidate in viral therapy for several tumors. VSV particles with mutated matrix (M) protein are capable of initiating cell death cascades while not being harmful to the immune system. In the current study, the effects of the VSV M-protein was investigated on the apoptosis of the colorectal cancer SW480 cell. Wild-type, M51R, and ΔM51 mutants VSV M-protein genes were cloned into the PCDNA3.1 vector and transfected into the SW480 cells. The results of the MTT assay, Western blotting, and Caspase 3, 8, and 9 measurement, illustrated that both wild and M51R mutant M-proteins can destroy the SW480 colorectal cancer cells. DAPI/TUNEL double-staining reconfirmed the apoptotic effects of the M-protein expression. The ΔM51 mutant M-protein is effective likewise M51R, somehow it can be considered as a safer substitution.





Similar content being viewed by others
References
Peterse EFP, Meester RGS, Siegel RL et al (2018) The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline: Young-Onset CRC: Screening Implications. Cancer 124:2964–2973. https://doi.org/10.1002/cncr.31543
Siegel RL, Miller KD, Fedewa SA et al (2017) Colorectal cancer statistics, 2017: colorectal Cancer Statistics, 2017. CA Cancer J Clin 67:177–193. https://doi.org/10.3322/caac.21395
Dolatkhah R, Somi MH, Kermani IA et al (2015) Increased colorectal cancer incidence in Iran: a systematic review and meta-analysis. BMC Public Health 15:997. https://doi.org/10.1186/s12889-015-2342-9
Japanese Society for Cancer of the Colon and Rectum, Watanabe T, Muro K et al (2018) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23:1–34. https://doi.org/10.1007/s10147-017-1101-6
Siegel RL, Miller KD, Jemal A (2017) Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. JAMA 318:572. https://doi.org/10.1001/jama.2017.7630
Frankel TL, D’Angelica MI (2014) Hepatic resection for colorectal metastases: hepatic resection for colorectal metastases. J Surg Oncol 109:2–7. https://doi.org/10.1002/jso.23371
Nagtegaal ID, Knijn N, Hugen N et al (2017) Tumor deposits in colorectal cancer: improving the value of modern staging—a systematic review and meta-analysis. J Clin Oncol 35:1119–1127. https://doi.org/10.1200/JCO.2016.68.9091
Guglielmo A, Staropoli N, Giancotti M, Mauro M (2018) Personalized medicine in colorectal cancer diagnosis and treatment: a systematic review of health economic evaluations. Cost Eff Resour Alloc 16:2. https://doi.org/10.1186/s12962-018-0085-z
Moore AE (1949) Effect of inoculation of the viruses of influenza A and herpes simplex on the growth of transplantable tumors in mice. Cancer 2:516–524. https://doi.org/10.1002/1097-0142(194905)2:3%3c516:AID-CNCR9%3e3.0.CO;2-P
Vile R, Ando D, Kirn D (2002) The oncolytic virotherapy treatment platform for cancer: unique biological and biosafety points to consider. Cancer Gene Ther 9:1062–1067. https://doi.org/10.1038/sj.cgt.7700548
Rowan K (2010) Oncolytic viruses move forward in clinical trials. JNCI J Natl Cancer Inst 102:590–595. https://doi.org/10.1093/jnci/djq165
Keene JD, Schubert M, Lazzarini RA, Rosenberg M (1978) Nucleotide sequence homology at the 3′ termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci USA 75:3225–3229. https://doi.org/10.1073/pnas.75.7.3225
Emerson SU, Wagner RR (1972) Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol 10:297–309
Quan B, Seo H-S, Blobel G, Ren Y (2014) Vesiculoviral matrix (M) protein occupies nucleic acid binding site at nucleoporin pair (Rae1bulletNup98). Proc Natl Acad Sci USA 111:9127–9132. https://doi.org/10.1073/pnas.1409076111
Ahmed M, Cramer SD, Lyles DS (2004) Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34–49. https://doi.org/10.1016/j.virol.2004.08.039
Ma Y-J, Yang J, Fan X-L et al (2012) Cellular microRNA let-7c inhibits M1 protein expression of the H1N1 influenza A virus in infected human lung epithelial cells. J Cell Mol Med 16:2539–2546. https://doi.org/10.1111/j.1582-4934.2012.01572.x
D’agostino PM, Amenta JJ, Reiss CS (2009) IFN-β-induced alteration of VSV protein phosphorylation in neuronal cells. Viral Immunol 22:353–369. https://doi.org/10.1089/vim.2009.0057
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296. https://doi.org/10.1146/annurev.immunol.25.022106.141609
Critchley-Thorne RJ, Simons DL, Yan N et al (2009) Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci USA 106:9010–9015. https://doi.org/10.1073/pnas.0901329106
Obuchi M, Fernandez M, Barber GN (2003) Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol 77:8843–8856. https://doi.org/10.1128/JVI.77.16.8843-8856.2003
Yamaki M, Shinozaki K, Sakaguchi T et al (2013) The potential of recombinant vesicular stomatitis virus-mediated virotherapy against metastatic colon cancer. Int J Mol Med 31:299–306. https://doi.org/10.3892/ijmm.2012.1205
Bishnoi S, Tiwari R, Gupta S et al (2018) Oncotargeting by vesicular stomatitis virus (VSV): advances in cancer therapy. Viruses 10:90. https://doi.org/10.3390/v10020090
Black BL, Rhodes RB, McKenzie M, Lyles DS (1993) The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J Virol 67:4814–4821
Heiber JF, Barber GN (2011) Vesicular stomatitis virus expressing tumor suppressor p53 is a highly attenuated, potent oncolytic agent. J Virol 85:10440–10450. https://doi.org/10.1128/JVI.05408-11
Felt SA, Droby GN, Grdzelishvili VZ (2017) Ruxolitinib and polycation combination treatment overcomes multiple mechanisms of resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus. J Virol 91:e00461-17. https://doi.org/10.1128/JVI.00461-17
Kopecky SA, Willingham MC, Lyles DS (2001) Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol 75:12169–12181. https://doi.org/10.1128/JVI.75.24.12169-12181.2001
Janelle V, Brassard F, Lapierre P et al (2011) Mutations in the glycoprotein of vesicular stomatitis virus affect cytopathogenicity: potential for oncolytic virotherapy. J Virol 85:6513–6520. https://doi.org/10.1128/JVI.02484-10
Gaddy DF, Lyles DS (2007) Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol 81:2792–2804. https://doi.org/10.1128/JVI.01760-06
Gaddy DF, Lyles DS (2005) Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol 79:4170–4179. https://doi.org/10.1128/JVI.79.7.4170-4179.2005
Cary ZD, Willingham MC, Lyles DS (2011) Oncolytic vesicular stomatitis virus induces apoptosis in U87 glioblastoma cells by a type II death receptor mechanism and induces cell death and tumor clearance in vivo. J Virol 85:5708–5717. https://doi.org/10.1128/JVI.02393-10
Hamacher R, Schmid RM, Saur D, Schneider G (2008) Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer 7:64. https://doi.org/10.1186/1476-4598-7-64
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Chen (2011) Cantharidin induces G2/M phase arrest and apoptosis in human colorectal cancer colo 205 cells through inhibition of CDK1 activity and caspase-dependent signaling pathways. Int J Oncol 38:1067–1073. https://doi.org/10.3892/ijo.2011.922
Chung J-G, Yang J-S, Huang L-J et al (2007) Proteomic approach to studying the cytotoxicity of YC-1 on U937 leukemia cells and antileukemia activity in orthotopic model of leukemia mice. Proteomics 7:3305–3317. https://doi.org/10.1002/pmic.200700200
Loo DT (2011) In situ detection of apoptosis by the TUNEL Assay: an overview of techniques. In: Didenko VV (ed) DNA damage detection in situ, ex vivo, and in vivo. Humana Press, Totowa, pp 3–13
Kyrylkova K, Kyryachenko S, Leid M, Kioussi C (2012) Detection of apoptosis by TUNEL assay. In: Kioussi C (ed) Odontogenesis. Humana Press, Totowa, pp 41–47
Scaffidi C, Schmitz I, Zha J et al (1999) Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem 274:22532–22538. https://doi.org/10.1074/jbc.274.32.22532
Publicover J, Ramsburg E, Robek M, Rose JK (2006) Rapid pathogenesis induced by a vesicular stomatitis virus matrix protein mutant: viral pathogenesis is linked to induction of tumor necrosis factor alpha. J Virol 80:7028–7036. https://doi.org/10.1128/JVI.00478-06
Raux H, Obiang L, Richard N et al (2010) The matrix protein of vesicular stomatitis virus binds dynamin for efficient viral assembly. J Virol 84:12609–12618. https://doi.org/10.1128/JVI.01400-10
Irie T, Liu Y, Drolet BS et al (2012) Cytopathogenesis of vesicular stomatitis virus is regulated by the PSAP motif of M protein in a species-dependent manner. Viruses 4:1605–1618. https://doi.org/10.3390/v4091605
von Kobbe C, van Deursen JMA, Rodrigues JP et al (2000) Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol Cell 6:1243–1252. https://doi.org/10.1016/S1097-2765(00)00120-9
Beilstein F, Obiang L, Raux H, Gaudin Y (2015) Characterization of the interaction between the matrix protein of vesicular stomatitis virus and the immunoproteasome subunit LMP2. J Virol 89:11019–11029. https://doi.org/10.1128/JVI.01753-15
Balachandran S, Barber GN (2004) Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51–65. https://doi.org/10.1016/S1535-6108(03)00330-1
Pearce AF, Lyles DS (2009) Vesicular stomatitis virus induces apoptosis primarily through Bak rather than Bax by inactivating Mcl-1 and Bcl-XL. J Virol 83:9102–9112. https://doi.org/10.1128/JVI.00436-09
Felt SA, Moerdyk-Schauwecker MJ, Grdzelishvili VZ (2015) Induction of apoptosis in pancreatic cancer cells by vesicular stomatitis virus. Virology 474:163–173. https://doi.org/10.1016/j.virol.2014.10.026
Roy S, Nicholson DW (2000) Cross-talk in cell death signaling. J Exp Med 192:F21–F26. https://doi.org/10.1084/jem.192.8.F21
Urbiola CR, Dold C, Kimpel J et al (2016) Abstract A191: augmenting the therapeutic efficacy of oncolytic LCMV-GP pseudotyped vesicular stomatitis virus via modulation of the innate immune system. Cancer Immunol Res 4:A191–A191. https://doi.org/10.1158/2326-6074.CRICIMTEATIAACR47-A191
Acknowledgements
The authors gratefully appreciate Mrs. Javid and Mr. Bazouri, research scientists at the Department of Microbiology, Golestan University of Medical Sciences (GoUMS) for their assistance in this work. This research was conducted at the laboratory of the Department of Microbiology as well as the Cell and Molecular Research Center, GoUMS and financially supported by the Deputy of Research and Technology, GoUMS, Gorgan, IR.
Author information
Authors and Affiliations
Contributions
ZG and MRK did the experiments, data analysis and text preparation; AT and AM did the project design, data analysis and discussion. AM was the PI of the project. All authors have seen, and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The project was ethically approval by the Ethics Committee of Golestan University of Medical Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gray, Z., Tabarraei, A., Moradi, A. et al. M51R and Delta-M51 matrix protein of the vesicular stomatitis virus induce apoptosis in colorectal cancer cells. Mol Biol Rep 46, 3371–3379 (2019). https://doi.org/10.1007/s11033-019-04799-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04799-3