Abstract
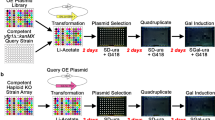
Zinc finger nuclease (ZFN) technology is a powerful molecular tool for targeted genome modifications and genetic engineering. However, screening for specific ZFs and validation of ZFN activity are labor intensive and time consuming. We previously designed a yeast-based ZFN screening and validation system by inserting a ZFN binding site flanked by a 164 bp direct repeat sequence into the middle of a Gal4 transcription factor, disrupting the open reading frame of the yeast Gal4 gene. Expression of the ZFN causes a double stranded break at its binding site, which promotes the cellular DNA repair system to restore expression of a functional Gal transcriptional factor via homologous recombination. Expression of Gal4 transcription factor leads to activation of three reporter genes in an AH109 yeast two-hybrid strain. However, the 164 bp direct repeat appears to generate spontaneous homologous recombination frequently, resulting in many false positive ZFNs. To overcome this, a series of DNA fragments of various lengths from 10 to 150 bp with 10 bp increase each and 164 bp direct repeats flanking the ZFN binding site were designed and constructed. The results demonstrated that the minimum length required for ZFN-induced homologous recombination was 30 bp, which almost eliminated spontaneous recombination. Using the 30 bp direct repeat sequence, ZFN could efficiently induce homologous recombination, while false positive ZFNs resulting from spontaneous homologous recombination were minimized. Thus, this study provided a simple, fast and sensitive ZFN screening and activity validation system in yeast.




Similar content being viewed by others
References
Cathomen T, Joung JK (2008) Zinc-finger nucleases: the next generation emerges. Mol Ther J Am Soc Gene Ther 16(7):1200–1207. doi:10.1038/mt.2008.114
Isalan M (2012) Zinc-finger nucleases: how to play two good hands. Nat Methods 9(1):32–34. doi:10.1038/nmeth.1805
Osakabe K, Osakabe Y, Toki S (2010) Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc Natl Acad Sci USA 107(26):12034–12039. doi:10.1073/pnas.1000234107
Tovkach A, Zeevi V, Tzfira T (2009) A toolbox and procedural notes for characterizing novel zinc finger nucleases for genome editing in plant cells. Plant J Cell Mol Biol 57(4):747–757. doi:10.1111/j.1365-313X.2008.03718.x
Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, Choi VM, Rock JM, Wu YY, Katibah GE, Zhifang G, McCaskill D, Simpson MA, Blakeslee B, Greenwalt SA, Butler HJ, Hinkley SJ, Zhang L, Rebar EJ, Gregory PD, Urnov FD (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459(7245):437–441. doi:10.1038/nature07992
Zhang F, Voytas DF (2011) Targeted mutagenesis in Arabidopsis using zinc-finger nucleases. Methods Mol Biol 701:167–177. doi:10.1007/978-1-61737-957-4_9
Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK, Voytas DF (2009) High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 459(7245):442–445. doi:10.1038/nature07845
Zhang F, Maeder ML, Unger-Wallace E, Hoshaw JP, Reyon D, Christian M, Li X, Pierick CJ, Dobbs D, Peterson T, Joung JK, Voytas DF (2010) High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc Natl Acad Sci USA 107(26):12028–12033. doi:10.1073/pnas.0914991107
Beumer K, Bhattacharyya G, Bibikova M, Trautman JK, Carroll D (2006) Efficient gene targeting in Drosophila with zinc-finger nucleases. Genetics 172(4):2391–2403. doi:10.1534/genetics.105.052829
Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Amacher SL (2008) Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol 26(6):702–708. doi:10.1038/nbt1409
Foley JE, Yeh JR, Maeder ML, Reyon D, Sander JD, Peterson RT, Joung JK (2009) Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN). PLoS ONE 4(2):e4348. doi:10.1371/journal.pone.0004348
Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R (2009) Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325(5939):433. doi:10.1126/science.1172447
Voigt B, Serikawa T (2009) Pluripotent stem cells and other technologies will eventually open the door for straightforward gene targeting in the rat. Dis Models Mech 2(7–8):341–343. doi:10.1242/dmm.002824
Lee HJ, Kim E, Kim JS (2010) Targeted chromosomal deletions in human cells using zinc finger nucleases. Genome Res 20(1):81–89. doi:10.1101/gr.099747.109
Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH (2008) Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol 26(7):808–816. doi:10.1038/nbt1410
Zou J, Maeder ML, Mali P, Pruett-Miller SM, Thibodeau-Beganny S, Chou BK, Chen G, Ye Z, Park IH, Daley GQ, Porteus MH, Joung JK, Cheng L (2009) Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells. Cell Stem Cell 5(1):97–110. doi:10.1016/j.stem.2009.05.023
Wright DA, Thibodeau-Beganny S, Sander JD, Winfrey RJ, Hirsh AS, Eichtinger M, Fu F, Porteus MH, Dobbs D, Voytas DF, Joung JK (2006) Standardized reagents and protocols for engineering zinc finger nucleases by modular assembly. Nat Protoc 1(3):1637–1652. doi:10.1038/nprot.2006.259
Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, Curtin SJ, Blackburn JS, Thibodeau-Beganny S, Qi Y, Pierick CJ, Hoffman E, Maeder ML, Khayter C, Reyon D, Dobbs D, Langenau DM, Stupar RM, Giraldez AJ, Voytas DF, Peterson RT, Yeh JR, Joung JK (2011) Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat Methods 8(1):67–69. doi:10.1038/nmeth.1542
Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, Unger-Wallace E, Sander JD, Muller-Lerch F, Fu F, Pearlberg J, Gobel C, Dassie JP, Pruett-Miller SM, Porteus MH, Sgroi DC, Iafrate AJ, Dobbs D, McCray PB Jr, Cathomen T, Voytas DF, Joung JK (2008) Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell 31(2):294–301. doi:10.1016/j.molcel.2008.06.016
Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA (2008) Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol 26(6):695–701. doi:10.1038/nbt1398
Fishman-Lobell J, Rudin N, Haber JE (1992) Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol 12(3):1292–1303
Lin FL, Sperle K, Sternberg N (1984) Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol 4(6):1020–1034
Lin FL, Sperle K, Sternberg N (1990) Repair of double-stranded DNA breaks by homologous DNA fragments during transfer of DNA into mouse L cells. Mol Cell Biol 10(1):113–119
Maryon E, Carroll D (1991) Characterization of recombination intermediates from DNA injected into Xenopus laevis oocytes: evidence for a nonconservative mechanism of homologous recombination. Mol Cell Biol 11(6):3278–3287
Sugawara N, Haber JE (1992) Characterization of double-strand break-induced recombination: homology requirements and single-stranded DNA formation. Mol Cell Biol 12(2):563–575
Ahn BY, Dornfeld KJ, Fagrelius TJ, Livingston DM (1988) Effect of limited homology on gene conversion in a Saccharomyces cerevisiae plasmid recombination system. Mol Cell Biol 8(6):2442–2448
Nickoloff JA, Singer JD, Hoekstra MF, Heffron F (1989) Double-strand breaks stimulate alternative mechanisms of recombination repair. J Mol Biol 207(3):527–541
Ray A, Siddiqi I, Kolodkin AL, Stahl FW (1988) Intra-chromosomal gene conversion induced by a DNA double-strand break in Saccharomyces cerevisiae. J Mol Biol 201(2):247–260
Rudin N, Haber JE (1988) Efficient repair of HO-induced chromosomal breaks in Saccharomyces cerevisiae by recombination between flanking homologous sequences. Mol Cell Biol 8(9):3918–3928
Rudin N, Sugarman E, Haber JE (1989) Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics 122(3):519–534
Ozenberger BA, Roeder GS (1991) A unique pathway of double-strand break repair operates in tandemly repeated genes. Mol Cell Biol 11(3):1222–1231
Fishman-Lobell J, Haber JE (1992) Removal of nonhomologous DNA ends in double-strand break recombination: the role of the yeast ultraviolet repair gene RAD1. Science 258(5081):480–484
Gysler-Junker A, Bodi Z, Kohli J (1991) Isolation and characterization of Schizosaccharomyces pombe mutants affected in mitotic recombination. Genetics 128(3):495–504
Perez C, Guyot V, Cabaniols JP, Gouble A, Micheaux B, Smith J, Leduc S, Paques F, Duchateau P (2005) Factors affecting double-strand break-induced homologous recombination in mammalian cells. Biotechniques 39(1):109–115
Ira G, Haber JE (2002) Characterization of RAD51-independent break-induced replication that acts preferentially with short homologous sequences. Mol Cell Biol 22(18):6384–6392
Sugawara N, Ira G, Haber JE (2000) DNA length dependence of the single-strand annealing pathway and the role of Saccharomyces cerevisiae RAD59 in double-strand break repair. Mol Cell Biol 20(14):5300–5309
Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39(12):e82. doi:10.1093/nar/gkr218
McCammon JM, Doyon Y, Amacher SL (2011) Inducing high rates of targeted mutagenesis in zebrafish using zinc finger nucleases (ZFNs). Methods Mol Biol 770:505–527. doi:10.1007/978-1-61779-210-6_20
Kleinstiver BP, Wolfs JM, Kolaczyk T, Roberts AK, Hu SX, Edgell DR (2012) Monomeric site-specific nucleases for genome editing. Proc Natl Acad Sci USA 109(21):8061–8066. doi:10.1073/pnas.1117984109
Epinat JC, Arnould S, Chames P, Rochaix P, Desfontaines D, Puzin C, Patin A, Zanghellini A, Paques F, Lacroix E (2003) A novel engineered meganuclease induces homologous recombination in yeast and mammalian cells. Nucleic Acids Res 31(11):2952–2962
Arnould S, Perez C, Cabaniols JP, Smith J, Gouble A, Grizot S, Epinat JC, Duclert A, Duchateau P, Paques F (2007) Engineered I-CreI derivatives cleaving sequences from the human XPC gene can induce highly efficient gene correction in mammalian cells. J Mol Biol 371(1):49–65. doi:10.1016/j.jmb.2007.04.079
Arnould S, Chames P, Perez C, Lacroix E, Duclert A, Epinat JC, Stricher F, Petit AS, Patin A, Guillier S, Rolland S, Prieto J, Blanco FJ, Bravo J, Montoya G, Serrano L, Duchateau P, Paques F (2006) Engineering of large numbers of highly specific homing endonucleases that induce recombination on novel DNA targets. J Mol Biol 355(3):443–458. doi:10.1016/j.jmb.2005.10.065
Gietz RD, Schiestl RH (2007) Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2(1):35–37. doi:10.1038/nprot.2007.14
Zhang T, Lei J, Yang H, Xu K, Wang R, Zhang Z (2011) An improved method for whole protein extraction from yeast Saccharomyces cerevisiae. Yeast 28(11):795–798. doi:10.1002/yea.1905
Wahls WP, Moore PD (1990) Homologous recombination enhancement conferred by the Z-DNA motif d(TG)30 is abrogated by simian virus 40 T antigen binding to adjacent DNA sequences. Mol Cell Biol 10(2):794–800
Wahls WP, Wallace LJ, Moore PD (1990) Hypervariable minisatellite DNA is a hotspot for homologous recombination in human cells. Cell 60(1):95–103
Ayares D, Chekuri L, Song KY, Kucherlapati R (1986) Sequence homology requirements for intermolecular recombination in mammalian cells. Proc Natl Acad Sci USA 83(14):5199–5203
Liskay RM, Letsou A, Stachelek JL (1987) Homology requirement for efficient gene conversion between duplicated chromosomal sequences in mammalian cells. Genetics 115(1):161–167
Shen P, Huang HV (1986) Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics 112(3):441–457
Singer BS, Gold L, Gauss P, Doherty DH (1982) Determination of the amount of homology required for recombination in bacteriophage T4. Cell 31(1):25–33
Jinks-Robertson S, Michelitch M, Ramcharan S (1993) Substrate length requirements for efficient mitotic recombination in Saccharomyces cerevisiae. Mol Cell Biol 13(7):3937–3950
Yuan J, Strack PR, Toniatti C, Pelletier M (2012) A zinc finger nuclease assay to rapidly quantitate homologous recombination proficiency in human cell lines. Anal Biochem. doi:10.1016/j.ab.2012.11.002
Connelly JP, Barker JC, Pruett-Miller S, Porteus MH (2010) Gene correction by homologous recombination with zinc finger nucleases in primary cells from a mouse model of a generic recessive genetic disease. Mol Ther J Am Soc Gene Ther 18(6):1103–1110. doi:10.1038/mt.2010.57
White CI, Haber JE (1990) Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J 9(3):663–673
Lambert S, Saintigny Y, Delacote F, Amiot F, Chaput B, Lecomte M, Huck S, Bertrand P, Lopez BS (1999) Analysis of intrachromosomal homologous recombination in mammalian cell, using tandem repeat sequences. Mutat Res 433(3):159–168
Hurt JA, Thibodeau SA, Hirsh AS, Pabo CO, Joung JK (2003) Highly specific zinc finger proteins obtained by directed domain shuffling and cell-based selection. Proc Natl Acad Sci USA 100(21):12271–12276. doi:10.1073/pnas.2135381100
Acknowledgments
We thank members of the Zhang Z lab for comments. This study was supported by Grants from National Natural Science Foundation of China (No. 31172186) and National Basic Research Program (No. 2011CBA01002).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, C., Yan, Q. & Zhang, Z. Minimum length of direct repeat sequences required for efficient homologous recombination induced by zinc finger nuclease in yeast. Mol Biol Rep 41, 6939–6948 (2014). https://doi.org/10.1007/s11033-014-3579-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3579-6