Abstract
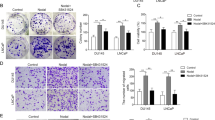
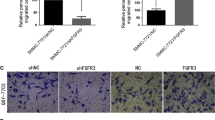
Hypoxia stimulates several pathways that are critical to cancer cell growth and survival, including activation of vascular endothelial growth factor (VEGF) transcription. Overexpression of VEGF and the extent of neoangiogenesis are closely correlated with tumor development and cancer metastases. Recent studies suggest MDM2 as one of the major regulators of pro-angiogenic mechanisms. To assess the direct correlation of HIF-1α and NF-κB, and the actual mechanism of MDM2 involved in the control over VEGF transcription, we exposed the LNCaP and LNCaP-MST cells (MDM2 transfected) to hypoxia. Our experiments confirm that MDM2 activation can lead to significant decrease in the levels of p53 in MDM2 transfected LNCaP-MST cells than the wild-type LNCaP cells. The results further suggest that MDM2 can be a strong regulator of both p53 dependent and independent transcriptional activity. Similarly, an increased level of other transcription factors such as HIF-1α, P300, STAT3, pAKT and NF-κB was observed. As a point of convergence for many oncogenic signaling pathways, STAT3 is constitutively activated at high frequency in a wide diversity of cancers. Our results indicate that STAT3 can directly regulate VEGF expression that is controlled by MDM2. Furthermore, it is evident from our results that NF-κB may interfere with the transcriptional activity of p53, by downregulating its levels. On the other hand, several pro-angiogenic mechanisms, including VEGF transcription which is controlled by MDM2, seem to be mediated by NF-κB.




Similar content being viewed by others
References
Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Herttuala SY, Alitalo K (2000) Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 21:4593–4599
Nugent MA, Lozzo RV (2000) Fibroblast growth factor-2. Int J Biochem Cell Biol 32:115–120
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD (1996) Isolation ofangiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87:1161–1169
Eliceiri BP, Cheresh DA (1999) The role of αv integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Investig 103:1227–1230
Roberts DD (1996) Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J 10:1183–1191
O’Reilly MS, Holmgren L, Chen SY, Rosenthal C, Cao RA, Moses Y, Lane WS, Sage EH, Folkman J (1994) Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb Symp Quant Biol 59:471–482
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285
Ferrera N (1999) Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56:794–814
Shibuya M (2006) Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 39:469–478
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Song Z, Guan B, Bergman A, Nicholson DW, Thornberry NA, Peterson EP, Steller H (2000) Biochemical and genetic interactions between Drosophila caspases and the proapoptotic genes rpr, hid, and grim. Mol Cell Biol 20:2907–2914
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Liao D, Johnson RS (2007) Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastas Rev 26:281–290
Narasimhan M, Rose R, Karthikeyan M, Rathinavelu A (2007) Detection of HDM2 and VEGF co-expression in cancer cell lines: novel effect of HDM2 antisense treatment on VEGF expression. Life Sci 81:1362–1372
Momand J, Zambetti GP (1997) MDM-2: “big brother’’ of p53. J Cell Biochem 64:343–352
Osman I, Sherman E, Singh B, Venkatraman E, Zelefsky M, Bosl G, Scher H, Shah J, Shaha A, Kraus D, Cordon-Cardo C, Pfister DS (2002) Alteration of p53 pathway in squamous cell carcinoma of the head and neck: impact on treatment outcome in patients treated with larynx preservation intent. J Clin Oncol 20:2980–2987
Leite JS, Martins SC, Oliveira J, Cunha MF, Castro-Sousa F (2011) Clinical significance of macroscopic completeness of mesorectal resection in rectal cancer. Colorectal Dis 13:381–386
Khor LY, DeSilvio M, Al-Saleem T, Hammond ME, Grignon DJ, Sause W, Pilepich M, Okunieff P, Sandler H, Pollack A (2005) MDM2 as a predictor of prostate carcinoma outcome. Cancer 104:962–967
Haupt Y, Maya R, Kazaz A, Oren M (1997) MDM2 promotes the rapid degradation of p53. Nature 387:296–299
Kubbutat HGM, Ludwig RL, Levine AJ, Vousden KH (1999) Analysis of the degradation function of Mdm2. Cell Growth Differ 10:87–92
Freedman DA, Wu L, Levine AJ (1999) Functions of the MDM2 oncoprotein. Cell Mol Life Sci 55:96–107
Oren M (1999) Regulation of the p53 tumor suppressor protein. J Biol Chem 274:36031–36034
Fakharzadeh SS, Trusko SP, George DL (1991) Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J 10:1565–1569
Jones SN, Roe AE, Donehower LA, Bradley A (1995) Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378:206–208
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Schuringa JJ, Schepers H, Vellenga E, Kruijer W (2001) Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett 495:71–76
Michael JG, Jing Z, Lee ME, Gregg LS, Douglas BE, Stephanie SW, Gary EG (2005) HIF-1α, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene 24:3110–3120
Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA (2001) Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA2 and p300/CBP. J Biol Chem 276:12645–12653
Baldwin AS (2001) Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J Clin Invest 107:241–246
Garg AK, Aggarwal BB (2002) Reactive oxygen intermediates in TNF signalling. Mol Immunol 39:509–517
Zhang J, Peng B (2009) NF-κB promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell Prolif 42:150–161
Karin AO, Dimberg A, Kreuger J, Welsh LC (2006) VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7:359–371
Rathinavelu P, Malave A, Raney SR, Hurst J, Roberson CT, Rathinavelu A (1999) Expression of mdm-2 oncoprotein in the primary and metastatic sites of mammary tumour (GI-101) implanted athymic nude mice. Cancer Biochem Biophys 17:133–146
Karin M, Greten FR (2005) 2NF-B: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5:749–759
Prashanth AK, Levine AJ (2010) p53 and NF-κB: different strategies for responding to stress lead to a functional antagonism. FASEB J 10:3643–3652
Bárdos JI, Ashcroft M (2004) Hypoxia-inducible factor-1 and oncogenic signalling. BioEssays 26:262–269
Carroll VA, Ashcroft M (2008) Regulation of angiogenic factors by HDM2 in renal cell carcinoma. Cancer Res 68:545–552
Narasimhan M, Rose R, Ramakrishnan R, Zell JA, Rathinavelu A (2008) Identification of HDM2 as a regulator of VEGF expression in cancer cells. Life Sci 82:1231–1241
Zhou Y, Li N, Zhuang W, Wu X (2011) Vascular endothelial growth factor (VEGF) gene polymorphisms and gastric cancer risk in a Chinese Han population. Mol Carcinog 50:184–188
Cao Y, Eble JM, Moon E, Yuan H, Weitzel DH, Landon CD, Nien CY, Hanna G, Rich JN, Provenzale JM, Dewhirst MW (2013) Tumor cells upregulate normoxic HIF-1α in response to doxorubicin. Cancer Res 73:6230–6242
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Busuttil V, Droin N, McCormicka L, Bernassolac F, Candi E, Melino G, Greena DR (2010) NF-κB inhibits T-cell activation-induced, p73-dependent cell death by induction of MDM2. Proc Natl Acad Sci USA 107:18061–18066
Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC (2000) Her-2/Neu blocks TNF-induced apoptosis by stimulating the transactivational potential of the RelA/p65 subunit of NF-κB. J Biol Chem 275:8027–8031
Rathinavelu A, Narasimhan M, Muthumani P (2011) Novel regulation of VEGF expression by HIF-1α and STAT3 in HDM2 transfected prostate cancer cells. J Cell Mol Med 16:1750–1757
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137:413–431
Farnebo M, Bykov VJN, Wiman KG (2010) The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun 396:85–89
Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB (1992) Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA 89:7491–7495
Lowe SW, Ruley HE, Jacks T, Housman DE (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957–967
Prives C, Hall PA (1999) The p53 pathway. J Pathol 187:112–126
Ryan KM, Phillips AC, Vousden KH (2001) Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol 13:332–337
Kubbutat MHG, Vousden KH (1998) Keeping an old friend under control: regulation of p53 stability. Mol Med Today 6:250–256
Tao W, Levine AJ (1999) P19ARF stabilizes p53 by blocking nucleo-cytoplasmic shuttling of MDM2. Proc Natl Acad Sci USA 96:6937–6941
Lo H, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC (2007) Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 67:9066–9076
Lau KW, Tian YM, Raval RR, Ratcliffe PJ, Pugh CW (2007) Target gene selectivity of hypoxia-inducible factor-α in renal cancer cells is conveyed by post-DNA-binding mechanisms. Br J Cancer 96:1284–1292
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Eli Keshet E (1998) Role of HIF-1 in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485–490
Mayo M, Baldwin A, Norris J (2001) Ras regulation of NF-κB and apoptosis. Methods Enzymol 333:73–87
Kortylewski M, Jove R, Yu H (2005) Targeting STAT3 affects melanoma on multiple fronts. Cancer Metast Rev 24:315–327
Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W et al (2011) PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov 1:158–169
Bonizzi G, Karin M (2004) The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25:280–288
Hayden MS, Ghosh S (2004) Signaling to NF-κB. Genes Dev 18:2195–2224
Kim S, Domon-Dell C, Kang J, Chung DH, Freund JN, Evers BM (2004) Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-alpha/nuclear factor-kappaB (NF-κB)-inducing kinase/NF-kappaB pathway is linked to a default kappa-alpha autoregulatory loop. J Biol Chem 279:4285–4291
Vasudevan KM, Gurumurthy S, Rangnekar VM (2004) Suppression of PTEN expression by NF-κB prevents apoptosis. Mol Cell Biol 24:1007–1021
Pahl HL (1999) Activators and target genes of Rel/NF-κB transcription factors. Oncog 22:6853–6866
Michael D, Oren M (2002) The p53 and MDM2 families in cancer. Curr Opin Genet Dev 12:53–59
Sherr AJ (2001) Parsing Ink4a/Arf: “Pure’’ p16-null mice. Cell 106:531–534
Rocha S, Campbell KJ, Perkins ND (2003) p53 and MDM2-independent repression of NF-κB transactivation by the ARF tumor suppressor. Mol Cell 12:15–25
Acknowledgments
We would like to thank Dr. Thomas Powell (Cleveland Clinic Foundation, Cleveland, OH, USA) and Dr. Alan Pollack (Fox Chase Cancer Center, Philadelphia, PA, USA) for their kind gift of LNCaP and LNCaP-MST cells, respectively. We acknowledge the Royal Dames of Cancer Research at Fort Lauderdale, Florida and President's Faculty Research and Development Grant (PFRDG) of Nova Southeastern University (NSU) for their support.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthumani, P., Alagarsamy, K., Dhandayuthapani, S. et al. Pro-angiogenic effects of MDM2 through HIF-1α and NF-κB mediated mechanisms in LNCaP prostate cancer cells. Mol Biol Rep 41, 5533–5541 (2014). https://doi.org/10.1007/s11033-014-3430-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3430-0