Abstract
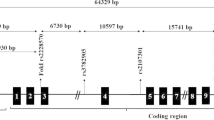
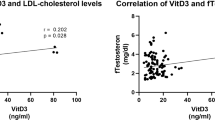
Obesity, insulin resistance, and hyperandrogenism are considered crucial parameters of polycystic ovary syndrome (PCOS) which might be related to vitamin D metabolism. The aim of this study was to investigate the associations between polymorphisms (TaqI and ApaI) in the vitamin D receptor gene (VDR) and PCOS among Egyptian women. We aimed also to elucidate the impact of these polymorphisms on vitamin D level, hormonal and metabolic parameters of PCOS. One hundred and fifty Egyptian women with PCOS and 150 unrelated controls were enrolled in this study. Polymorphisms of VDR Taq-I T/C (rs731236) and Apa-I A/C (rs7975232) gene were genotyped using polymerase chain reaction restriction fragment length polymorphism (PCR–RFLP). Serum 25 hydroxy vitamin D [25(OH) D] levels were measured by high-performance liquid chromatography. PCOS women had significantly lower levels of 25(OH) D compared to healthy women. Our results revealed that Taq-I CC genotype and C allele were associated with increased risk of PCOS, while the Apa-I polymorphism was not. Haplotype Taq-I C/ Apa-I C was associated with a higher PCOS risk more than controls. Moreover, there was a significant decrease of 25(OH) D levels in carriers of haplotype Taq-I C/ Apa-I C (with variant alleles) compared to the non-carriers. Results showed also that there was an obesity- VDR Taq-I genotypes interactions. These results suggested that, VDR Taq-I gene polymorphism is associated with increased risk of PCOS in Egyptian women.


Similar content being viewed by others
References
Asuncio´n M, Calvo RM, San Milla´n JL, Sancho J, Avila S , Escobar- Morreale HF (2000) A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 85(7):2434–2438
Teede H, Deeks A, Moran L (2010) Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impact on health across the lifespan. BMC Med 30:8–41
Mahmoudi T (2009) Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril 92(4):1381–1383
Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann, K, Janssen OE (2006) Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 114(10):577–583
Weber RF, Pache TD, Jacobs ML, Docter R, Loriaux DL, Fauser BC, Birkenhäger JC (1993) The relation between clinical manifestations of polycystic ovary syndrome and beta-cell function. Clin Endocrinol (Oxf) 38(3):295–300
Aronne LJ, Segal KR (2002) Adiposity and fat distribution outcome measures: assessment and clinical implications. Obes Res 10(1):14–21
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R (2002) Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 26(7):883–896
Dale PO, Tanbo T, Vaaler S, Abyholm T (1992) Body weight, hyperinsulinemia, and gonadotropin levels in the polycystic ovarian syndrome: evidence of two distinct populations. Fertil Steril 58:487–491
Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z (2010) Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril 93(4):1208–1214
Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R (2008) Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr 47:87–91
Chiu KC, Chuang LM, Yoon C (2001) The vitamin D receptor polymorphism in the translation initiation codon is a risk factor for insulin resistance in glucose tolerant Caucasians. BMC Med Genet 2:2
Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW (1998) The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13:325–349
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike JW, Shine J, O’Malley BW (1988) Cloning the expression of full length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA 85:3294–3298
DeLuca HF (2004) Therapeutic potential of the 2-alkyl and 2-alkylidene-19-nor-20S-modified analogs of 1a, 25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 89(90):67–73
Maestro B, Da´vila N, Carranza MC & Calle C (2003) Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 84:223–230
Ogunkolade BW, Boucher BJ, Prahl JM, Bustin SA, Burrin JM, Noonan K et al (2002) Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 51:2294–2300
The Rotterdam ESHRE-ASRM-sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19(1):41–47
National Institutes of Health Consensus Development Panel (1998) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obes Res 6(2):51–209
Frieldewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Turpeinen U, Hohenthal U, Stenman UH (2003) Determination of 25-hydroxyvitamin D in serum by HPLC and immunoassay. Clin Chem 49(9):1521–1524
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
Ranjzad F, Mahban A, Shemirani AI, Mahmoudi T, Vahedi M, Nikzamir A, Zali MR (2011) Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet 28(3):225–232
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96(1):53
Rashidi B, Haghollahi F, Shariat M, Zayerii F (2009) The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol 48(2):142–147
Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK (2011) Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 60(10):1475–1481
Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR et al (2009) Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol 161(4):575–582
Panidis D, Balaris C, Farmakiotis D, Rousso D, Kourtis A, Balaris V, Katsikis I, Zournatzi V, Diamanti-Kandarakis E (2005) Serum parathyroid hormone concentration are increased in women with polycystic ovary syndrome. Clin Chem 51(9):1691–1697
Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP (1999) Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 6:430–435
Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, Kamaci M (2009) Serum 25-hydroxyvitamin D concentrations in obese and non obese women with polycystic ovary syndrome. Arch Gynecol Obstet 280(4):559–563
Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM, Lam AC, Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Tuomilehto-Wolf E et al (2004) Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet 13:1633–1639
Zofkova I, Scholz G, Starka L (1989) Effect of calcitonin and 1, 25 (OH)2-vitamins D3 on the FSH, LH and testosterone secretion at rest and LHRH stimulated secretion. Horm Metab Res 21:682–685
Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook P N, Eisman JA (1994) Prediction of bone density from vitamin D receptor alleles. Nature 367:284–287
Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B (2011) Vitamin D–associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol 164(5):741–749
Muscogiuri G, Sorice GP, Prioletta A, Policola C, Della Casa S, Pontecorvi A, Giaccari A (2010) 25-Hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 18:1906–1910
Pasquali R, Casimirri F, Vicennati V (1997) Weight control and its beneficial effect on fertility in women with obesity and polycystic ovary syndrome. Hum Reprod 1:82–87
Ye WZ, Reis AF, Dubois-Laforgue D, Bellanné-Chantelot C, Timsit J, Velho G (2001) Vitamin D receptor gene polymorphisms are associated with obesity in Type 2 diabetic subjects with early age of onset. Eur J Endocrinol 145:181–186
Kamei Y, Kawada T, Kazuki R, Ono T, Kato S ,Sugimoto E (1993) Vitamin D receptor gene expression is up-regulated by 1,25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 193:948–955
Lenoir C, Dace A, Martin C, Bonne J, Teboul M, Planells R et al (1996) Calcitriol down-modulates the 3,5,30 triiodothyronine (T3) receptors and affects, in a biphasic manner, the T3-dependent adipose differentiation of Ob 17 preadipocytes. Endocrinology 137:4268–4276
Dace A, Martin-El Yazidi C, Bonne J, Planells R, Torresani J (1997) Calcitriol is a positive effector of adipose differentiation in the OB 17 cell line: relationship with the adipogenic action of triiodothyronine. Biochem Biophys Res Commun 232:771–776
Kawada T, Kamei Y, Sugimoto E (1996) The possibility of active form of vitamins A and D as suppressors on adipocyte development via ligand-dependent transcriptional regulators. Int J Obes Relat Metab Disord 20(3):52–57
Santos BR, Mascarenhas LP, Satler F, Boguszewski MC, Spritzer PM (2012) Vitamin D deficiency in girls from South Brazil: a cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr 8(12):62
Maestro B, Molero S, Bajo S, Da´vila N, Calle C (2002) Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct 20:227–232
Bikle D (2009) Nonclassic actions of vitamin D. J Clin Endocrinol Metab 94:26–34
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169–2180
Acknowledgments
The authors wish to express their sincere thanks to all volunteers and patients.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Shal, A.S., Shalaby, S.M., Aly, N.M. et al. Genetic variation in the vitamin D receptor gene and vitamin D serum levels in Egyptian women with polycystic ovary syndrome. Mol Biol Rep 40, 6063–6073 (2013). https://doi.org/10.1007/s11033-013-2716-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2716-y