Abstract
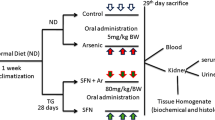
Arsenic (As) is an environmental and industrial pollutant that affects various organs in human and experimental animals. Silibinin is a naturally occurring plant bioflavonoid found in the milk thistle of Silybum marianum, which has been reported to have a wide range of pharmacological properties. A body of evidence has accumulated implicating the free radical generation with subsequent oxidative stress in the biochemical and molecular mechanisms of As toxicity. Since kidney is the critical target organ of chronic As toxicity, we carried out this study to investigate the effects of silibinin on As-induced toxicity in the kidney of rats. In experimental rats, oral administration of sodium arsenite [NaAsO2, 5 mg/(kg day)] for 4 weeks significantly induced renal damage which was evident from the increased levels of serum urea, uric acid, creatinine with a significant (p < 0.05) decrease in creatinine clearance. As also significantly decreased the levels of urea, uric acid and creatinine in urine. A markedly increased levels of lipid peroxidation markers (thiobarbituric acid reactive substances and lipid hydroperoxides) and protein carbonyl contents with significant (p < 0.05) decrease in non-enzymatic antioxidants (total sulfhydryl groups, reduced glutathione, vitamin C and vitamin E) and enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxidase and glutathione S-transferase), Glutathione metabolizing enzymes (glutathione reductase and glutathione-6-phosphate dehydrogenase) and membrane bound ATPases were also observed in As treated rats. Co-administration of silibinin (75 mg/kg day) along with As resulted in a reversal of As-induced biochemical changes in kidney accompanied by a significant decrease in lipid peroxidation and an increase in the level of renal antioxidant defense system. The histopathological and immunohistochemical studies in the kidney of rats also shows that silibinin (75 mg/kg day) markedly reduced the toxicity of As and preserved the normal histological architecture of the renal tissue, inhibited the caspase-3 mediated tubular cell apoptosis and decreased the NADPH oxidase, iNOS and NF-κB over expression by As and upregulated the Nrf2 expression in the renal tissue. The present study suggests that the nephroprotective potential of silibinin in As toxicity might be due to its antioxidant and metal chelating properties, which could be useful for achieving optimum effects in As-induced renal damage.










Similar content being viewed by others
Change history
07 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11033-021-06475-x
References
Yadav RS, Sankhwar ML, Shukla RK, Chandra R, Pant AB, Islam F, Khanna VK (2009) Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol Appl Pharmacol 240:367–376
Ratnaike RN (2003) Acute and chronic arsenic toxicity. Postgrad Med J 79:391–396
Ginsburg JM (1965) Renal mechanism for excretion and transformation of arsenic in the dog. Am J Physiol 208:832–840
Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP (2000) Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci 55:460–467
Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, Liang B, Lin J, Liu Y, Liu B, Zhou Q, Zhang D, Wan R, Ma N, Su X, Niu K, Pe Y, Xu W, Chen Z, Wan H, Cui J, Barhanin J, Chen Y (2004) Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet 75:899–905
Lin A, Zhang X, Zhu YG, Zhao FJ (2008) Arsenate-induced toxicity: effects on antioxidative enzymes and DNA damage in Vicia faba. Environ Toxicol Chem 2:4139
Li Y, Ling M, Xu Y, Wang S, Li Z, Zhou J, Wang X, Liu Q (2010) The repressive effect of NF-kappaB on p53 by mot-2 is involved in human keratinocyte transformation induced by low levels of arsenite. Toxicol Sci 116:174–182
Taylor DJ, Green NPO, Stout GW, Soper R (2003) Biological sciences. Cambridge University Press, Cambridge, pp 680–692
Bhadauria S, Flora SJS (2007) Response of arsenic-induced oxidative stress, DNA damage and metal imbalance to combined administration of DMSA and monoisoamyl-DMSA during chronic arsenic poisoning in rats. Cell Biol Toxicol 23:91–104
Kokilavani V, Devi MA, Sivarajan K, Panneerselvam C (2005) Combined efficacies of DL-ά-Lipoic acid and meso 2,3-dimercaptosuccinic acid against arsenic induced toxicity in antioxidant system of rats. Toxicol Lett 160:1–7
Uivarosi V, Barbuceanu SF, Aldea V, Arama CC, Badea M, Olar R, Marinescu D (2010) Synthesis, spectral and thermal studies of new rutin vanadyl complexes. Molecules 15:1578–1589
Sakai K, Li Y, Shirakawa T, Kitagawa Y, Hirose G (2001) Induction of major histocompatibility complex I class molecules on human neuroblastoma line cells by a flavonoid antioxidant. Neurosci Lett 298:127–130
Zhou B, Wu LJ, Tashiro S, Onodera S, Uchiami F, Ikejima T (2006) Silibinin protects rat cardiac myocyte from isoproterenol-induced DNA damage independent on regulation on regulation of cell cycle. Biol Pharmacol Bull 29:1900–1905
Dietzmann J, Thiel U, Ansorge S, Neumann KH, Tager M (2002) Thiol-inducing and immunoregulatory effects of flavonoids in perinuclear blood mononuclear cells from patients with end-stage diabetic nephropathy. Free Radic Biol Med 33:1347–1354
Ligeret H, Brault A, Vellerand D, Hadded Y, Hadded PS (2008) Antioxidant and mitochondrial protective effects of silibinin in cold preservation warm reperfusion liver injury. J Ethnopharmacol 115:507–514
Niehiaus WG, Samuelsson D (1968) Formation of malondialdehyde from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
Jiang ZY, Hunt JV, Wolff SD (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Ann Biochem 202:384–389
Levine RL, Garland D, Oliver CN, Amic A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Moron MS, Despierre JW, Minnervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Omaye ST, Turnbull JD, Sauberlich HE (1979) Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol 62:3–11
Desai ID (1984) Vitamin E analysis method for animal tissues. Methods Enzymol 105:138–143
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Sinha AK (1972) Colorimetric assay of catalase. Ann Biochem 47:389–394
Rotruck JT, Rope AL, Ganther HF, Swason AB (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Habig WH, Pabst MJ, Jakpoly WB (1974) Glutathione transferase: a first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Horn HD, Burns FH (1978) Assay of glutathione reductase activity. In: Bergmeyer HV (ed) Methods of enzymatic analysis. Academic Press, New York, pp 142–146
Beutler E (1983) Active transport of glutathione disulfide from erythrocytes. In: Larson A, Orrenius S, Holmgren A, Mannerwik B (eds) Functions of glutathione, biochemical, physiological, toxicological and clinical aspects. Raven Press, New York, p 65
Lowry OH, Rosenbrough MJ, Farr AL, Randall RJ (1951) Protein measurement with Folin–phenol reagent. J Biol Chem 193:265–275
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Evans DJ Jr (1969) Membrane adenosine triphosphatase of E. coli activation by calcium ions and inhibition by monovalent cations. J Bacteriol 100:914–922
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Bonting SL (1970) Presence of enzyme system in mammalian tissues. In: Bilter EE (ed) Membrane and ion transport. Wiley Inter Science, London, pp 257–263
Hjerten S, Pan H (1983) Purification and characterization of two forms of low affinity Ca++–ATPase from erythrocyte membrane. Biochim Biophys Acta 728:281–288
Ohnishi T, Suzuki T, Suzuki Y, Ozawa K (1982) A comparative study of plasma membrane Mg++–ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta 684:67–74
Shi H, Djikeng A, Tschudi C, Ullu E (2004) Argonaut protein in the early divergent eukaryote Trypanosoma brucei: control of small interfering RNA accumulation and retroposon transcript abundance. Mol Cell Biol 24:420–427
Sinha H, Manna P, Sil PC (2008) Arjunolic acid attenuates arsenic-induced nephrotoxicity. Pathophysiology 15:147–156
Muthumani M, Milton Prabu S (2012) Silibinin potentially protects arsenic induced oxidative hepatic dysfunction in rats. Toxicol Mech Methods 22:277–288
Rajnarayana K, Sripalreddy M, Chaluvadi MR, Krishna DR (2001) Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol 33:2–16
Patel HR, Kalia K (2010) Sub-chronic arsenic exposure aggravates nephrotoxicity in experimental diabetic rats. Indian J Exp Biol 48:762–768
Basiglio CL, Sanchez Pozzi EJ, Mottino AD, Roma MG (2009) Differential effects of silymarin and its active component SB on plasma membrane stability and hepatocellular lysis. Chem Biol Interact 179:297–303
Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV (2004) Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci 101:4578–4583
Usoh IF, Akpan EJ, Etim EO, Farombi EO (2005) Antioxidant actions of dried flower extracts of Hibiscus sabdariffa L. on sodium arsenite-induced oxidative stress in rats. Pakistan J Nutr 4:135–141
Yamanaka K, Hasegawa A, Sawamura R, Okada S (1991) Cellular response to oxidative damage in lung induced by administration of dimethyl arsenic acid, a major metabolite of inorganic arsenic in mice. Toxicol Appl Pharmacol 108:205–213
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Kirkman MN, Gaetani GF (1984) Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc Nat Acad Sci USA 81:4343–4347
Danyelle TM, Kenneth TD (2003) The role of glutathione S-transferase in anticancer drug resistance. Drug Res 22:7369–7375
Flora SJS, Kannan GM, Kumar P (1999) Selenium effects on gallium arsenite induced biochemical and immunotoxicological changes in rat. Chem Biol Interact 122:1–13
Ganyc D, Talbot S, Konate F, Jackson S, Schanen B, Cullen W, Self WT (2007) Impact of trivalent arsenicals on selenoprotein synthesis. Environ Health Perspect 115:346–353
Shila S, Kokilavani V, Subathra M (2005) Brain regional responses in antioxidant system to α-lipoic acid in arsenic intoxicated rat. Toxicology 210:25–36
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Haddad Y, Vallerand D, Brault A, Haddad PS (2011) Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid Based Complement Alternat Med. doi:10.1093/ecam/nep164
Sharmila Banu G, Kumar G, Murugesan AG (2009) Ethanolic leaf extract of Trianthema portulacastrum L. A meliorates aflatoxin B1 induced hepatic damage in rats. Indian J Clin Biochem 24:250–256
Kempaiah RK, Srinivasan K (2006) Protective effect of curcumin, capsaicin and garlic on erythrocyte integrity in high fat fed rats. J Nutr Biochem 17:471–478
Ithayarasi AP, Devi CS (1997) Effect of alpha-tocopherol on lipid peroxidation in isoproterenol induced myocardial infarction in rats. Indian J Physiol Pharmacol 41:369–376
Finotti P, Palatini P (1986) Reduction of erythrocyte Na+/K+ ATPase activity in type I insulin dependent diabetic subjects and its activation by homologus plasma. Diabetology 29:623–628
Das AK, Bag S, Sahu R, Dua TK, Sinha MK, Gangopadhyay M (2010) Protective effect of Corchorus olitorius leaves on sodium arsenite-induced toxicity in experimental rats. Food Chem Toxicol 48:326–335
Katiyar SK (2005) Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects (review). Int J Oncol 26:169–176
Bratton SB, Cohen GM (2001) Apoptotic death sensor: an organelle’s alter ego? Trends Pharmacol Sci 22:306–315
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Prabu, S.M., Muthumani, M. RETRACTED ARTICLE: Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep 39, 11201–11216 (2012). https://doi.org/10.1007/s11033-012-2029-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2029-6