Abstract
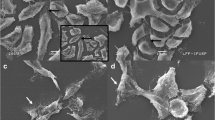
Because of its lower toxicity and good tolerability and response, gemcitabine has been described as one of the most highly promising drugs for urinary bladder cancer therapy. Its phosphorylated active-dFdCTP metabolite can incorporate into DNA, causing replication blockage. Additionally, it is known that mutations in the TP53 gene are related to the high recurrence rate of these neoplasias. Based on these premises, we investigated the effects of gemcitabine on the expression of the cell cycle-related genes in two different TP53-mutated bladder transitional carcinoma cell lines–5637 (from a moderate-grade tumor with a TP53 allele carrying two mutations) and T24 (from an invasive tumor with a TP53 allele encoding an in-frame deletion). Cell viability and morphology analyses (phase-contrast photomicrographs), Nuclear Division Index and pathway-specific quantitative RT-PCR gene arrays were performed. Treatment with gemcitabine led to the following results: (1) a significant decrease of viable T24 cells after treatment at the highest concentration (3.12 μM) tested; (2) scattered, elongated and vacuolated 5637 and T24 cells; (3) a cytostatic effect in both cell lines; and (4) significant upregulation of the BRCA1, CCNE1, CDK2, CDK6, CDKN1A, CDKN2B, E2F4, GADD45A, MAD2L2, CCNH, SERTAD1, CDC1, and CHEK1 genes. Gemcitabine had distinct toxicogenomic effects in the bladder transitional carcinoma cell lines with two different TP53 mutations. However, independent of the type of mutation and tumor grade, gemcitabine induced cell cycle arrest; upregulation of DNA repair-related genes, G1/S transition, apoptosis and activation of transcription factors, mainly by upregulation of the CCNE1, CDKN1A and GADD45A genes.



Similar content being viewed by others
References
Cordon-Cardo C (2008) Molecular alterations associated with bladder cancer initiation and progression. Scand J Urol Nephrol 218:154–165
Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI, Breinl E, Merseburger AS, Shariat SF (2011) Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol 29:457–463
Cheng L, Zhang S, MacLennan GT, Williamson SR, Lopez-Beltrn A, Montironi R (2011) FRCPath and IFCAP. Bladder cancer: translating molecular genetic insights into clinical practice. Human Pathol 42:455–481
Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DWW (2006) Predicting recurrence and progression in individual patients with stage TaT1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49:466–477
Nishiyama H, Watanabe J, Ogawa O (2008) p53 and chemosensitivity in bladder cancer. Int J Clin Oncol 13:282–286
Fujita H, Ohuchida K, Mizumoto K, Itaba S, Ito T, Nakata K, Yu J, Kayashima T, Souzaki R, Tajiri T, Manabe T, Ohtsuka T, Tanaka M (2010) Gene expression levels as predictive markers of outcome in pancreatic cancer after gemcitabine-based adjuvant chemotherapy. Neoplasia 12:807–817
Castagneto B, Zai S, Marenco D, Bertetto O, Repetto L, Scaltriti L, Mencoboni M, Ferraris V, Botta M (2004) Single-agent gemcitabine in previously untreated elderly patients with advanced bladder carcinoma: response to treatment and correlation with the comprehensive geriatric assessment. Oncology 67:27–32
Bellmut J, Albiol S, de Olano AR, Pujadas J, Maroto P (2006) On behalf the Spanish Oncology Genitourinary Group (SOGUG). Gemcitabine in the treatment of advanced transitional cell carcinoma of the urothelium. Ann Oncol 17:113–117
Shelley MD, Cleves A, Wilt TJ, Mason MD (2011) Gemcitabine chemotherapy for the treatment of metastatic bladder carcinoma. BJU Int 108:168–179
Toschi L, Finocchiaro G, Gioia V (2005) Role of gemcitabine in cancer therapy? Future Oncol 1:7–17
Ruiz Van Haperen VWT, Veerman G, Vermoken JB, Peters GJ (1993) 2′,2′-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA from tumor cell lines. Biochem Pharmacol 46:762–766
Gontero P, Frea B (2006) Actual experience and future development of gemcitabine in superficial bladder cancer. Ann Oncol 17:123–128
Achanta G, Pelicano H, Feng L, Plunkett W (2001) Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res 61:8723–8729
Bergman AM, Pinedo HM, Peters GJ (2002) Determinants of resistance to 2′,2′- difluorodeoxycytidine (gemcitabine). Drug Resist Updat 5:19–33
Galmarini CM, Clarke ML, Falette N, Puisieux A, Mackey JR, Dumontet C (2002) Expression of a non-functional p53 affects the sensitivity of cancer cells to gemcitabine. Int J Cancer 97:439–445
da Silva GN, Marcondes JPC, Camargo EA, Sakamoto-Hojo ET, Passos GA, Salvadori DMF (2010) Cell cycle arrest and apoptosis in TP53 subtypes of bladder carcinoma cell lines treated with cisplatin and gemcitabine. Exp Med Biol 235:814–824
Toshimitsu H, Iizuka N, Yamamoto K, Kawauchi S, Oga A, Furuya T, Oka M, Sasaki K (2006) Molecular features linked to the growth-inhibitory effects of gemcitabine on human pancreatic cancer cells. Oncol Rep 16:1285–1291
Pourquier P, Gioffre C, Kohlhagen G, Urasaki Y, Goldwasser F, Hertel LW (2002) Gemcitabine (20,20-difluoro-20-deoxycytidine), an antimetabolite that poisons topoisomerase I. Clin Cancer Res 8:2499–2504
Pearce HL, Miller MA (2005) The evolution of cancer research and drug discovery at Lilly, Research Laboratories. Adv Enzyme Regul 45:229–255
Coppée J-Y (2008) Do DNA microarrays have their future behind them? Microbes Infect 10:1067–1071
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104
Fencher G, Perabo FGE, Schmidt DH, Haase L, Ludwig E, Schueller H, Blatter J, Muller C, Albers P (2003) Preclinical evaluation of a radiosensitizing effect of gemcitabine in p53 mutant and p53 wild type bladder cancer cells. Urology 61:468–473
Aydemir N, Celikler S, Bilaloglu R (2005) In vitro genotoxic effect of the anticancer drug gemcitabine in human lymphocytes. Mutat Res 582:35–41
Jiang HT, Hickey RJ, Abdel-Aziz W, Malkas LH (2000) Effects of gemcitabine and araC on in vitro DNA synthesis mediated by the human breast cell DNA synthesome. Cancer Chemother Pharmacol 45:320–328
Attene-Ramos MS, Nava GM, Muellner MG, Wagner ED, Plewa MJ, Gaskins HR (2010) DNA damage and toxicogenomic analyses of Hydrogen Sulfide in human intestinal epithelial FHs 74 Int cells. Environ Mol Mutagen 51:304–314
Li G-C, Zhang X, Pan T-J, Chen Z, Ye Z-Q (2006) Histone deacetylase inhibitor trichostatin A inhibits the growth of bladder cancer cells through induction of p21waf1 and G1 cell cycle arrest. Int J Urol 13:581–586
Li T-M, Chen G-W, Su C-C, Lin J-G, Yeh C-C, Cheng K-C, Chung J-G (2005) Ellagic Acid Induced p53/p21 Expression, G1 Arrest and Apoptosis in Human Bladder Cancer T24 Cells. Anticancer Res 25:971–980
Missiaglia E, Donadelli M, Palmieri M, Crnogorac-Jurcevic T, Scarpa A, Lemoine NR (2005) Growth delay of human pancreatic cancer cells by methylase inhibitor 5-aza-2′-deoxycytidine treatment is associated with activation of the interferon signalling pathway. Oncogene 24:199–211
Mazumder D, Mitra S, Singh RK, Dutta S, Roy A, Mondal RK, Basu PS, Roychoudhury S, Panda CK (2011) Inactivation of CHEK1 and EI24 is associated with the development of invasive cervical carcinoma: clinical and prognostic implications. Int J Cancer 129:1859–1871
Pleska D, Crosby ME, Gupta D, Almasan A (2007) E2F4 function in G2: maintaining G2-arrest to prevent mitotic entry with damaged DNA. Cell Cycle 6:1147–1152
Galizia E, Giorgetti G, Piccinini G, Santinelli A, Loretelli C, Bianchi F, Gagliardini D, Carbonari G, Pisa E, Belvederesi L, Bracci R, Ferretti C, Corradini F (2010) BRCA1 expression in triple negative sporadic breast cancers. Anal Quant Cytol Histol 32:24–29
Hernandes-Vargas HH, Rodrigues-Pinilla SM, Julian-Tendero M, Sanchez-Rovira P, Cuevas C, Anton A, Rios MJ, Palacios J, Moreno Bueno G (2007) Gene expression profiling of breast cancer cells in response to gemcitabine: nK-kB pathway activation as a potential mechanism of resistance. Breast Cancer Res Treat 102:157–172
Cordon-Cardo C, Dalbagni G, Saez GT, Oliva MR, Zhang ZF, Rosai J, Reuter VE, Pellicer A (1994) p53 mutations in human bladder cancer: genotypic versus phenotypic patterns. Int J Cancer 56:347–353
Soria G, Gottifredi V (2010) PCNA-coupled p21 degradation after DNA damage: the exception that confirms the rule? DNA Repair 9:358–364
Lockwood WW, Stack D, Morris T, Grehan D, O’Keane C, Stewart GL, Cumiskey J, Lam WL, Squire JA, Thomas DM, O’Sullivan MJ (2011) Cyclin E1 is amplified and overexpressed in osteosarcoma. J Mol Diagn 13:289–296
Jiang J, Yang ES, Jian G (2011) Susceptibility to DNA damage p53-dependent BRCA1 nuclear export controls cellular. Cancer Res 71:5546–5557
Beukelaers P, Vandenbosch R, Caron N, Nguyen L, Belachew S, Moonen G, Kiyokawa H, Barbacid M, Santamaria D, Malgrange B (2011) Cdk6-dependent regulation of G(1) length controls adult neurogenesis. Stem Cells 29:713–724
Liu J, Vasudevan S, Kipreos ET (2004) CUL-2 and ZYG-11 promote meiotic anaphase II and the proper placement of the anterior-posterior axis in C. elegans. Development 131:3513–3525
Redwood AB, Gonzalez-Suarez I, Gonzalo S (2011) Regulating the levels of key factors in cell cycle and DNA repair: new pathways revealed by lamins. Cell Cycle 10:3652–3657
Soussi T, Wiman KG (2007) Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell 12:303–312
da Silva GN, Evangelista AF, Magalhães DA, Macedo C, Búfalo MC, Sakamoto-Hojo ET, Passos GA, Salvadori DM (2011) Expression of genes related to apoptosis, cell cycle and signaling pathways are independent of TP53 status in urinary bladder cancer cells. Mol Biol Rep 38:4159–4170
Bie L, Zhao G, Ju Y, Zhang B (2011) Integrative genomic analysis identifies CCNB1 and CDC2 as candidate genes associated with meningioma recurrence. Cancer Genet 204:536–540
de Angelis PM, Fjell B, Kravik KL, Haug T, Tunheim SH, Reichelt W, Beigi M, Clausen OP, Galteland E, Stokke T (2004) Molecular characterizations of derivatives of HCT116 colorectal cancer cells that are resistant to the chemotherapeutic agent 5-fluorouracil. Int J Oncol 24:1279–1288
Di Stefano AB, Iovino F, Lombardo Y, Eterno V, Höger T, Dieli F, Stassi G, Todaro M (2010) Survivin is regulated by interleukin-4 in colon cancer stem cells. J Cell Physiol 225:261–555
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL (2011) Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11:558–572
Acknowledgments
This study was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Glenda Nicioli da Silva and Elaine Aparecida de Camargo contributed equally to this study.
Rights and permissions
About this article
Cite this article
da Silva, G.N., de Camargo, E.A. & Salvadori, D.M.F. Toxicogenomic activity of gemcitabine in two TP53-mutated bladder cancer cell lines: special focus on cell cycle-related genes. Mol Biol Rep 39, 10373–10382 (2012). https://doi.org/10.1007/s11033-012-1916-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1916-1