Abstract
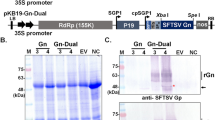
Influenza A viruses expose two major surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Although N-glycosylation is essential for many glycoproteins, the glycoproteins expressed in yeast are sometimes hyper-glycosylated, which maybe a primary hindrance to the exploitation of therapeutic glycoprotein production because glycoproteins decorated with yeast-specific glycans are immunogenic and show poor pharmacokinetic properties in humans. To elucidate the NA with different glycosylation in interaction with immunogenicity, here we reported the heterologous expression of influenza NA glycoprotein derived from influenza virus A/newCaledonia/20/99(H1N1) in wide-type Pichia pastoris, α-1,6-mannosyltransferase (och1)-defective P. pastoris and Escherichia coli. We also assessed the immunogenicity of hyper-glycosylated NA expressed in the wide-type, low-glycosylated NA expressed in och1-defective P. pastoris strain and non-glycosylated NA produced in E. coli. Recombinant NA was expressed in wide-type P. pastoris as a 59–97 above kDa glycoprotein, 52–57 kDa in the och1 defective strain, and as a 45 kDa non-glycoprotein in E. coli. The antibody titers of Balb/c mice were tested after the mice were immunized three times with 0.2, 1, or 3 μg purified recombinant NA. Our results demonstrated that after the second immunization, the antibody titer elicited with 1 μg low-glycosylated NA was 1:5,500, while it was 1:10 and 1:13 when elicited by 1 μg hyper-glycosylated and non-glycosylated NA. In the 0.2 μg dose groups, a high antibody titer (1:4,900) was only found after third immunization by low-glycosylated NA, respectively. These results suggest that low-glycosylation in och1-defective P. pastoris enhances the immunogenicity of recombinant NA and elicits similar antibody titers with less antigen when compared with hyper- and non-glycosylated NA. Thus, och1-defective P. pastoris may be a better yeast expression system for production of glycoproteins to research immunogenic characterization.



Similar content being viewed by others
Abbreviations
- Al(OH)3 :
-
Aluminium hydroxide
- HA:
-
Hemagglutinin
- NA:
-
Neuraminidase
- OCH1:
-
α-1,6-mannosyltransferase
- PNGase F:
-
Peptide-N-glycosidase F
- Man:
-
Mannose
- GlcNAc:
-
N-acetylglucosamine
References
Enserink M, Cohen J (2009) Virus of the year. The novel H1N1 influenza. Science 326(5960):1607. doi:10.1126/science.326.5960.1607
Rappuoli R, Del Giudice G, Nabel GJ, Osterhaus AD, Robinson R, Salisbury D, Stohr K, Treanor JJ (2009) Public health. Rethinking influenza. Science 326(5949):50. doi:10.1126/science.1179475
Hay AJ, Gregory V, Douglas AR, Lin YP (2001) The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci 356(1416):1861–1870. doi:10.1098/rstb.2001.0999
Schweiger B, Zadow I, Heckler R (2002) Antigenic drift and variability of influenza viruses. Med Microbiol Immunol 191(3–4):133–138. doi:10.1007/s00430-002-0132-3
Skehel J (2009) An overview of influenza haemagglutinin and neuraminidase. Biologicals 37(3):177–178. doi:10.1016/j.biologicals.2009.02.012
Schulman JL, Khakpour M, Kilbourne ED (1968) Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol 2(8):778–786
Johansson BE, Bucher DJ, Kilbourne ED (1989) Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol 63(3):1239–1246
Deroo T, Jou WM, Fiers W (1996) Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine 14(6):561–569
Martinet W, Saelens X, Deroo T, Neirynck S, Contreras R, Min JW, Fiers W (1997) Protection of mice against a lethal influenza challenge by immunization with yeast-derived recombinant influenza neuraminidase. Eur J Biochem 247(1):332–338
Saelens X, Vanlandschoot P, Martinet W, Maras M, Neirynck S, Contreras R, Fiers W, Jou WM (1999) Protection of mice against a lethal influenza virus challenge after immunization with yeast-derived secreted influenza virus hemagglutinin. Eur J Biochem 260(1):166–175
Johansson BE, Brett IC (2007) Changing perspective on immunization against influenza. Vaccine 25(16):3062–3065. doi:10.1016/j.vaccine.2007.01.030
Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, Holden-Wiltse J, Liang H, Gilbert A, Cox M (2007) Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA 297(14):1577–1582. doi:10.1001/jama.297.14.1577
Audsley JM, Tannock GA (2008) Cell-based influenza vaccines: progress to date. Drugs 68(11):1483–1491
Brands R, Visser J, Medema J, Palache AM, van Scharrenburg GJ (1999) Influvac: a safe Madin Darby Canine Kidney (MDCK) cell culture-based influenza vaccine. Dev Biol Stand 98:93–100; discussion 111
Romanova J, Katinger D, Ferko B, Vcelar B, Sereinig S, Kuznetsov O, Stukova M, Erofeeva M, Kiselev O, Katinger H, Egorov A (2004) Live cold-adapted influenza A vaccine produced in Vero cell line. Virus Res 103(1–2):187–193. doi:10.1016/j.virusres.2004.02.032
Kistner O, Howard MK, Spruth M, Wodal W, Bruhl P, Gerencer M, Crowe BA, Savidis-Dacho H, Livey I, Reiter M, Mayerhofer I, Tauer C, Grillberger L, Mundt W, Falkner FG, Barrett PN (2007) Cell culture (Vero) derived whole virus (H5N1) vaccine based on wild-type virus strain induces cross-protective immune responses. Vaccine 25(32):6028–6036. doi:10.1016/j.vaccine.2007.05.013
Shahsavandi S, Salmanian AH, Ghorashi SA, Masoudi S, Fotouhi F, Ebrahimi MM (2010) Specific subtyping of influenza A virus using a recombinant hemagglutinin protein expressed in baculovirus. Mol Biol Rep. doi:10.1007/s11033-010-0434-2
Ebrahimi SM, Tebianian M (2010) Heterologous expression, purification and characterization of the influenza A virus M2e gene fused to Mycobacterium tuberculosis HSP70 (359–610) in prokaryotic system as a fusion protein. Mol Biol Rep 37(6):2877–2883. doi:10.1007/s11033-009-9846-2
Li HZ, Gang HY, Sun QM, Liu X, Ma YB, Sun MS, Dai CB (2004) Production in Pichia pastoris and characterization of genetic engineered chimeric HBV/HEV virus-like particles. Chin Med Sci J 19(2):78–83
Bazan SB, de Alencar Muniz ChavesA, Aires KA, Cianciarullo AM, Garcea RL, Ho PL (2009) Expression and characterization of HPV-16 L1 capsid protein in Pichia pastoris. Arch Virol 154(10):1609–1617. doi:10.1007/s00705-009-0484-8
Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22(4):249–270. doi:10.1002/yea.1208
Sreekrishna K, Potenz RH, Cruze JA, McCombie WR, Parker KA, Nelles L, Mazzaferro PK, Holden KA, Harrison RG, Wood PJ et al (1988) High level expression of heterologous proteins in methylotrophic yeast Pichia pastoris. J Basic Microbiol 28(4):265–278
Grinna LS, Tschopp JF (1989) Size distribution and general structural features of N-linked oligosaccharides from the methylotrophic yeast, Pichia pastoris. Yeast 5(2):107–115. doi:10.1002/yea.320050206
Buckholz RG, Gleeson MA (1991) Yeast systems for the commercial production of heterologous proteins. Biotechnology (NY) 9(11):1067–1072
Wang Y, Gong X, Chang SH, Liu B, Song M, Huang HH, Wu J (2007) A Pichia pastoris with alpha-1,6-mannosyltransferases deletion and its use in the expression of HSA/GM-CSF chimera. Chin J Biotechnol 23(5):907–914. doi:10.1016/s1872-2075(07)60056-9
Yang YL, Chang SH, Gong X, Liu B, Wu J (2009) Prokaryotic expression, purification and immunogenicity of H1N1 influenza virus neuraminidase. Lett Biotechnol 20(6):757–760. doi:10.3969/j.issn.1009-0002.2009.06.002
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y
Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, Miele RG, Nett JH, Wildt S, Gerngross TU (2003) Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc Natl Acad Sci USA 100(9):5022–5027. doi:10.1073/pnas.0931263100
Yongkiettrakul S, Boonyapakron K, Jongkaewwattana A, Wanitchang A, Leartsakulpanich U, Chitnumsub P, Eurwilaichitr L, Yuthavong Y (2009) Avian influenza A/H5N1 neuraminidase expressed in yeast with a functional head domain. J Virol Methods 156(1–2):44–51. doi:10.1016/j.jviromet.2008.10.025
Deng MJ, Xiao XZ, Zhang YM, Wu XH, Zhu LH, Xin XQ, Wu DL (2011) A highly sensitive immuno-PCR assay for detection of H5N1 avian influenza virus. Mol Biol Rep 38(3):1941–1948. doi:10.1007/s11033-010-0315-8
Khurana S, Verma S, Verma N, Crevar CJ, Carter DM, Manischewitz J, King LR, Ross TM, Golding H (2010) Properly folded bacterially expressed H1N1 hemagglutinin globular head and ectodomain vaccines protect ferrets against H1N1 pandemic influenza virus. PLoS One 5 (7): e11548. doi:10.1371/journal.pone.0011548
Park YI, Wood HA, Lee YC (1999) Monosaccharide compositions of Danaus plexippus (monarch butterfly) and Trichoplusia ni (cabbage looper) egg glycoproteins. Glycoconj J 16(10):629–638
Kubelka V, Altmann F, Kornfeld G, Marz L (1994) Structures of the N-linked oligosaccharides of the membrane glycoproteins from three lepidopteran cell lines (Sf-21, IZD-Mb-0503, Bm-N). Arch Biochem Biophys 308(1):148–157. doi:10.1006/abbi.1994.1021
Kuroda K, Geyer H, Geyer R, Doerfler W, Klenk HD (1990) The oligosaccharides of influenza virus hemagglutinin expressed in insect cells by a baculovirus vector. Virology 174(2):418–429
Parker GF, Williams PJ, Butters TD, Roberts DB (1991) Detection of the lipid-linked precursor oligosaccharide of N-linked protein glycosylation in Drosophila melanogaster. FEBS Lett 290(1–2):58–60
Johansson BE, Price PM, Kilbourne ED (1995) Immunogenicity of influenza A virus N2 neuraminidase produced in insect larvae by baculovirus recombinants. Vaccine 13(9):841–845
Ghaffar A, Khan SA, Mukhtar Z, Rajoka MI, Latif F (2010) Heterologous expression of a gene for thermostable xylanase from Chaetomium thermophilum in Pichia pastoris GS115. Mol Biol Rep. doi:10.1007/s11033-010-9996-2
Cai X, Wang J, Wang Y, Yang Y, Gao J, Fu W, Xu D (2010) Expression, purification and characterization of recombinant human interleukin-22 in Pichia pastoris. Mol Biol Rep 37(6):2609–2613. doi:10.1007/s11033-009-9785-y
Acknowledgments
This work has been supported by the National High Technology Research and Development Program of China (863 Program) (No. 2007AA02Z103), the National Natural Science Foundation of China (NSFC) (No. 30870050), the Scientific and Technological Major Special Project—“Significant Creation of New Drugs” (No. 2009ZX09031-002) and the Beijing Natural Science Foundation (No. 5102037).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, YL., Chang, SH., Gong, X. et al. Expression, purification and characterization of low-glycosylation influenza neuraminidase in α-1,6-mannosyltransferase defective Pichia pastoris . Mol Biol Rep 39, 857–864 (2012). https://doi.org/10.1007/s11033-011-0809-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0809-z