Abstract
Spermatogenesis is a transitionary process in which the diploid spermatogonia transform into haploid mature spermatozoa. Actin and myosin have been implicated in various aspects during spermatogenesis. Actin is present in the form of monomer, oligomer and polymer within cells, the latter is called microfilament. There are five actin-containing structures during spermatogenesis, i.e., ectoplasmic specialization, acroplaxome, manchette in mammals, actin cones in Drosophila and acroframosome in Caridean shrimp. They are involved in the shaping and differentiating of spermatids. Along with spermatogenesis, the actin cytoskeletons show active remodeling in this process. Some actin binding or actin regulated proteins have been demonstrated to regulate dynamic changes of the actin-containing structures. Myosin, actin-dependent molecular motor, plays an important role during spermatogenesis, such as involving in acrosome biogenesis, vesicle transport, gene transcription and nuclear shaping. The actin cytoskeleton and actin binding/regulated proteins cooperate to facilitate spermatogenesis. In this review, we summarize the existing knowledge about the cytoskeletal structures consisting of actin, actin binding/regulated proteins and myosin during spermatogenesis.



Similar content being viewed by others
References
Clermont Y (1972) Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52:198–236
Pudney J (1995) Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech 32:459–497
Chen M, Shen X (2007) Nuclear actin and actin-related proteins in chromatin dynamics. Curr Opin Cell Biol 19:326–330
Pederson T, Aebi U (2002) Actin in the nucleus: what form and what for? J Struct Biol 140:3–9
Pederson T, Aebi U (2005) Nuclear actin extends, with no contraction in sight. Mol Biol Cell 16:5055–5060
Percipalle P, Visa N (2006) Molecular functions of nuclear actin in transcription. J Cell Biol 172:967–971
Zheng B, Han M, Bernier M, Wen JK (2009) Nuclear actin and actin-binding proteins in the regulation of transcription and gene expression. FEBS J 276:2669–2685
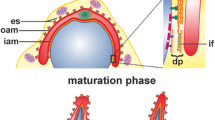
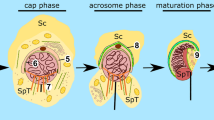
Kierszenbaum AL, Rivkin E, Tres LL (2003) Acroplaxome, an F-actin-keratin-containing plate, anchors the acrosome to the nucleus during shaping of the spermatid head. Mol Biol Cell 14:4628–4640
Noguchi T, Miller KG (2003) A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development 130:1805–1816
Lee NP, Cheng CY (2004) Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update 10:349–369
Yan HH, Mruk DD, Lee WM, Cheng CY (2006) Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays 29:36–48
Li Z, Pan CY, Zheng BH, Xiang L, Yang WX (2010) Immunocytochemical studies of the acroframosome during spermiogenesis of the caridean shrimp Macrobrachium nipponense (Crustacea, Natantia). Invertebr Reprod Dev 54(3):121–131
Mruk DD, Cheng CY (2010) Tight junctions in the testis: new perspectives. Philos Trans R Soc Lond B Biol Sci 365:1621–1635
Wong EW, Mruk DD, Cheng CY (2008) Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta 1778:692–708
Kierszenbaum AL, Tres LL (2004) The acrosome–acroplaxome–manchette complex and the shaping of the spermatid head. Arch Histol Cytol 67:271–284
Kierszenbaum AL (2002) Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev 63:1–4
Krendel M, Mooseker MS (2005) Myosins: tails (and heads) of functional diversity. Physiology 20:239–251
DePina AS, Langford GM (1999) Vesicle transport: the role of actin filaments and myosin motors. Microsc Res Tec 47:93–106
Howes EA, Hurst SM, Jones R (2001) Actin and actin-binding proteins in bovine spermatozoa: potential role in membrane remodeling and intracellular signaling during epididymal maturation and the acrosome reaction. J Androl 22:62–72
Murthy K, Wadsworth P (2005) Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol 15:724–731
Watanabe N, Mitchison TJ (2002) Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science 295:1083–1086
Cheng YM, Shi XQ, Yu H, Wu Y, Jia MC (2005) Specific expression of β-actin during spermatogenesis in rats. Zhonghua Nan Ke Xue 11:755–760
Weber K, Sokac A, Berg JS, Cheney RE, Bement WM (2004) A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature 431:325–329
Clubb BH, Locke M (1996) F-actin forms transient perinuclear shells at the mitosis-interphase transition. Cell Motil Cytoskeleton 33:151–162
Xiao X, Yang WX (2007) Actin-based dynamics during spermatogenesis and its significance. Zhejiang Univ Sci B 8:498–506
Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J (2000) Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol 63:1–15
Cheng CY, Mruk DD (2002) Cell junction dynamics in the testis: Sertoli germ cell interactions and male contraceptive development. Physiol Rev 82:825–874
Wong CH, Cheng CY (2005) The blood–testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol 71:263–296
Hermo L, Pelletier RM, Cyr DG, Smith CE (2010) Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: intercellular junctions and contacts between germs cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc Res Tech 73:409–494
Lie PP, Mruk DD, Lee WM, Cheng CY (2010) Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365:1581–1592
Cheng CY, Mruk DD (2009) An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 44:245–263
Setchell BP (2008) Blood–testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol 636:212–233
Anahara R, Toyama Y, Maekawa M, Kai M, Ishino F, Toshimori K, Mori C (2006) Flutamide depressed expression of cortactin in the ectoplasmic specialization between the Sertoli cells and spermatids in the mouse testis. Food Chem Toxicol 44:1050–1056
Bartles JR, Wierda A, Zheng L (1996) Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci 109:1229–1239
Lie PPY, Mruk DD, Lee WM, Cheng CY (2009) Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood–testis barrier integrity in the seminiferous epithelium. FASEB J 23:2555–2567
Guttman JA, Janmey P, Vogl AW (2002) Gelsolin-evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J Cell Sci 115:499–505
Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F, Di Fiore PP, Ciliberto A, Stradal TE, Scita G (2006) Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol 8:1337–1347
Grove BD, Vogl AW (1989) Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J Cell Sci 93:309–323
Vitale ML, Akpovi CD, Pelletier RM (2009) Cortactin/tyrosine-phosphorylated cortactin interaction with connexin 43 in mouse seminiferous tubules. Microsc Res Tech 72:856–867
Mruk DD, Lau AS (2009) RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol Reprod 80:590–601
Sun S, Wong EW, Li MW, Lee WM, Cheng CY (2009) 14-3-3 and its binding partners are regulators of protein–protein interactions during spermatogenesis. J Endocrinol 202:327–336
Wong EW, Sun S, Li MW, Lee WM, Cheng CY (2009) 14-3-3 Protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology 150:4713–4723
Wong EW, Mruk DD, Lee WM, Cheng CY (2008) Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood–testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci 105:9657–9662
Hasson T, Walsh J, Cable J, Mooseker MS, Brown SD, Steel KP (1997) Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton 37:127–138
Velichkova M, Guttman J, Warren C, Eng L, Kline K, Vogl AW, Hasson T (2002) A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil Cytoskeleton 51:147–164
Yazama F (2008) Continual maintenance of the blood–testis barrier during spermatogenesis: the intermediate compartment theory revisited. J Reprod Dev 54:299–305
Kierszenbaum AL (2006) Tyrosine protein kinases and spermatogenesis: truncation matters. Mol Reprod Dev 73:399–403
Kierszenbaum AL, Tres LL, Rivkin E, Kang-Decker N, van Deursen JM (2004) The acroplaxome is the docking site of Golgi-derived myosin Va/Rab27a/b- containing proacrosomal vesicles in wild-type and Hrb mutant mouse spermatids. Biol Reprod 70:1400–1410
Kierszenbaum AL, Rivkin E, Tres LL (2003) The actin-based motor myosin Va is a component of the acroplaxome, an acrosome-nuclear envelope junctional plate, and of manchette-associated vesicles. Cytogenet Genome Res 103:337–344
Jockusch BM, Murk K, Rothkegel M (2007) The profile of profilins. Rev Physiol Biochem Pharmacol 159:131–149
Yarmola EG, Bubb MR (2006) Profilin: emerging concepts and lingering misconceptions. Trends Biochem Sci 31:197–205
Behnen M, Murk K, Kursula P, Cappallo-Obermann H, Rothkegel M, Kierszenbaum AL, Kirchhoff C (2009) Testis-expressed profilins 3 and 4 show distinct functional characteristics and localize in the acroplaxome–manchette complex in spermatids. BMC Cell Biol doi:10.1186/1471-2121-10-34
Kierszenbaum AL, Rivkin E, Tres LL (2008) Expression of Fer testis (FerT) tyrosine kinase transcript variants and distribution sites of FerT during the development of the acrosome–acroplaxome–manchette complex in rat spermatids. Dev Dyn 237:3882–3891
Hara Y, Yamagata K, Oguchi K, Baba T (2008) Nuclear localization of profilin III-ArpM1 complex in mouse spermiogenesis. FEBS Lett 582:2998–3004
Clermont Y, Oko R, Hermo L (1993) Cell Biology of Mammalian Spermiogenesis. In: Desjardins C, Ewing L (eds) Cell and molecular biology of the testis. Oxford University Press, New York, pp 332–376
Hayasaka S, Terada Y, Suzuki K, Murakawa H, Tachibana I, Sankai T, Murakami T, Yaegashi N, Okamura K (2008) Intramanchette transport during primate spermiogenesis: expression of dynein, myosin Va, motor recruiter myosin Va, VIIa-Rab27a/b interacting protein, and Rab27b in the manchette during human and monkey spermiogenesis. Asian J Androl 10:561–568
Soley JT (1997) Nuclear morphogenesis and the role of the manchette during spermiogenesis in the ostrich (Struthio camelus). J Anat 190:563–576
Fabrizio JJ, Hime G, Lemmon SK, Bazinet C (1998) Genetic dissection of sperm individualization in Drosophila melanogaster. Development 125:1833–1843
Rogat AD, Miller KG (2002) A role for myosin VI in actin dynamics at sites of membrane remodeling during Drosophila spermatogenesis. J Cell Sci 115:4855–4865
Noguchi T, Lenartowska M, Rogat AD, Frank DJ, Miller KG (2008) Proper cellular reorganization during Drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Mol Biol Cell 19:2363–2372
Tokuhiro K, Miyagawa Y, Tanaka H (2008) Characterizing mouse male germ cell-specific actin capping protein alpha3 (CPalpha3): dynamic patterns of expression in testicular and epididymal sperm. Asian J Androl 10:711–718
Texada MJ, Simonette RA, Johnson CB, Deery WJ, Beckingham KM (2008) Yuri gagarin is required for actin, tubulin and basal body functions in Drosophila spermatogenesis. J Cell Sci 121:1926–1936
Mermall V, Bonafé N, Jones L, Sellers JR, Cooley L, Mooseker MS (2005) Drosophila myosin V is required for larval development and spermatid individualization. Dev Biol 286:238–255
Noguchi T, Lenartowska M, Miller KG (2006) Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol Biol Cell 17:2559–2571
Yang WX, Du NS, Lai W (1998) Changes of Golgi apparatus during spermatogenesis of Macrobrachium nipponense. Acta Zool Sin 44:377–383
Baker JP, Titus MA (1998) Myosins: matching motors with functions. Curr Opin Cell Biol 10:80–86
Mermall V, Post PL, Mooseker MS (1998) Unconventional myosins in cell movement, membrane traffic. Science 279:527–533
Tuxworth RI, Titus MA (2000) Unconventional myosins: anchors in the membrane traffic relay. Traffic 1:11–18
Abou-Haila A, Tulsiani DR (2000) Mammalian sperm acrosome: formation, contents, and function. Arch Biochem Biophys 379:173–182
Rosenblatt J, Cramer LP, Baum B, McGee KM (2004) Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 117:361–372
Woolner S, O’Brien LL, Wiese C, Bement WM (2008) Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol 182:77–88
Wühr M, Mitchison TJ, Field CM (2008) Mitosis: new roles for myosin-X and actin at the spindle. Curr Biol 18:912–914
Sun X, He Y, Hou L, Yang WX (2010) Myosin Va participates in acrosomal formation and nuclear morphogenesis during spermatogenesis of Chinese mitten crab Eriocheir sinensis. PLoS One 5(9):e12738. doi:10.1371/journal.pone.0012738
Buss F, Luzio JP, Kendrick-Jones J (2002) Myosin VI, an actin motor for membrane traffic and cell migration. Traffic 3:851–858
Kelleher JF, Mandell MA, Moulder G, Hill KL, L’Hernault SW, Barstead R, Titus MA (2000) Myosin VI is required for asymmetric segregation of cellular components during C. elegans spermatogenesis. Curr Biol 10:1489–1496
Bettinger BT, Glibert DM, Amberg DC (2004) Actin up in the nucleus. Nat Rev Mol Cell Biol 5:410–415
Olave IA, Reck-Peterson SL, Crabtree GR (2002) Nuclear actin and actin-related proteins in chromatin remodeling. Annu Rev Biochem 71:755–781
Fomproix N, Percipalle P (2004) An actin-myosin complex on actively transcribing genes. Exp Cell Res 294:140–148
Philimonenko VV, Zhao J, Iben S (2004) Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat Cell Biol 6:1165–1172
Vreugde S, Ferrai C, Miluzio A, Hauben E, Marchisio PC, Crippa MP, Bussi M, Biffo S (2006) Nuclear myosin VI enhances RNA polymerase II-dependent transcription. Mol Cell 23:749–755
Pranchevicius MC, Baqui MM, Ishikawa-Ankerhold HC, Lourenço EV, Leão RM, Banzi SR, dos Santos CT, Roque-Barreira MC, Espreafico EM, Larson RE (2008) Myosin Va phosphorylated on Ser1650 is found in nuclear speckles and redistributes to nucleoli upon inhibition of transcription. Cell Motil Cytoskeleton 65:441–456
Bookwalter CS, Lord M, Trybus KM (2009) Essential features of the class V myosin from budding yeast for ASH1 mRNA transport. Mol Biol Cell 20:3414–3421
Krauss J, López de Quinto S, Nüsslein-Volhard C, Ephrussi A (2009) Myosin-V regulates oskar mRNA localization in the Drosophila oocyte. Curr Biol 19:1058–1063
Salerno VP, Calliari A, Provance DW Jr, Sotelo-Silveira JR, Sotelo JR, Mercer JA (2008) Myosin-Va mediates RNA distribution in primary fibroblasts from multiple organs. Cell Motil Cytoskeleton 65:422–433
O’Brien DA (1987) Stage-specific protein synthesis by isolated spermatogenic cells throughout meiosis and early spermiogenesis in the mouse. Biol Reprod 37:147–157
Tanaka H, Baba T (2005) Gene expression in spermiogenesis. Cell Mol Life Sci 62:344–354
Wong EW, Cheng CY (2009) Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol 278:309–353
Acknowledgment
Grant sponsor National Natural Science Foundation of China, Grant No. 31072198 and 40776079; National Basic Research Program of China (973 Program), Grant No. 2007CB948104.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao Sun and Tamas Kovacs contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, X., Kovacs, T., Hu, YJ. et al. The role of actin and myosin during spermatogenesis. Mol Biol Rep 38, 3993–4001 (2011). https://doi.org/10.1007/s11033-010-0517-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0517-0