Abstract
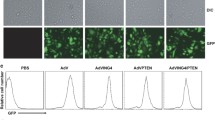
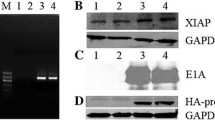
Data have increasingly shown that melanoma differentiation associated gene-7 (Mda-7/IL-24) has growth suppression activity and can induce apoptosis in many tumor cells, but to our knowledge there have been few studies about its role in colon cancer. We examined its anti-cancer effect on colon cancer. We constructed a recombinant replication-deficient adenovirus carrying human melanoma differentiation associated gene-7 (Ad-IL-24) and examined its apoptosis-inducing efficacy on the colon cancer HT-29 cell line and on an oxaliplatin-resistant cell line HT-29/oxa, using a combination of flow cytometry, growth suppressive activity by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and xenografts. Furthermore, we tested the suppression activity of Mda-7/IL-24 on vascular endothelial growth factor (VEGF) and microvessel density (MVD), as well as the inductive effect on expression of the growth arrest and DNA damage gene (GADD) in xenograft tumors by immunohistochemistry. Melanoma differentiation associated gene-7 can inhibit the growth of colon cancer cell lines and induced apoptosis in about (5.6 ± 0.3)% of HT-29 cells (P < 0.05). Xenograft growth was retarded in vivo in mice treated with melanoma differentiation associated gene-7, but the tumor proliferation rate for this group was not significantly different in comparison to controls (P > 0.05). Furthermore, melanoma differentiation associated gene-7 induced expression of a growth arrest and DNA damage (GADD) gene and reduced the expression of both VEGF and MVD in xenograft tumors. This study supports a potential therapeutic effect for melanoma differentiation associated gene-7 on colon cancer.







Similar content being viewed by others
References
Kotenko SV (2002) The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev 13:223–240
Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB (2004) Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 22:929–979
Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB (1995) Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 11:2477–2486
Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L et al (2006) mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther 111:596–628
Madireddi MT, Su ZZ, Young CS, Goldstein NI, Fisher PB (2000) Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol 465:239–261
Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB (1996) The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA 93:9160–9165
Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB (2002) The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene 21:708–718
Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C et al (2003) Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene 22:1164–1180
Fathallah-Shaykh HM, Zhao LJ, Kafrouni AI, Smith GM, Forman J (2000) Gene transfer of IFN-γ into established brain tumors represses growth by antiangiogenesis. J Immunol 164:217–222
Angiolillo AL, Sgadari C, Tosato G (1996) A role for the interferon-inducible protein 10 in inhibition of angiogenesis by interleukin-12. Ann N Y Acad Sci 795:158–167
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS et al (1997) An endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 5:2509–2514
Krajewska M, Kim H, Kim C, Kang H, Welsh K, Matsuzawa S et al (2005) Analysis of apoptosis protein expression in early-stage colorectal cancer suggests opportunities for new prognostic biomarkers. Clin Cancer Res 11:5451–5461
Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M et al (2002) Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 38:1564–1579
Liebermann DA, Hoffman B (2002) Myeloid differentiation (MyD)/growth arrest DNA damage (GADD) genes in tumor suppression, immunity and inflammation. Leukemia 16:527–541
Sun L, Gong R, Wan B et al (2003) GADD45gamma, down-regulated in 65% hepatocellular carcinoma (HCC) from 23 Chinese patients, inhibits cell growth and induces cell cycle G2/M arrest for hepatoma Hep-G2 cell lines. Mol Biol Rep 30:249–253
Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K et al (2002) Md-7(IL-24) mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci USA 99:10054–10059
Lee JC, Chow NH, Wang ST, Huang SM (2002) Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 36:748–753
Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM (1995) Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 55:3964–3968
Wong MP, Cheung N, Yuen ST, Leung SY, Chung LP (1999) Vascular endothelial growth factor is up-regulated in the early pre-malignant stage of colorectal tumour progression. Int J Cancer 81:845–850
Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K et al (2003) Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res 63:5105–5113
Acknowledgments
This work was supported by grants from the Outstanding Medical Academic Program of Jiang Su Province, China (NO.RC2007076).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, S., Yang, J., Chen, W. et al. Antitumor activity of an adenovirus harboring human IL-24 in colon cancer. Mol Biol Rep 38, 395–401 (2011). https://doi.org/10.1007/s11033-010-0121-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0121-3