Abstract
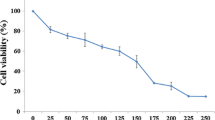
Many lines of evidence have shown that Chinese medicine contains many chemical compounds with anticancer effects. Therefore, we tested whether the active ingredients of blister beetles have a therapeutic effect on hepatoma. The aim of this study was to investigate the inhibitive effects of norcantharidin which is extracted from blister beetles on human hepatoma cells HepG2 in vitro and its anticancer mechanism.MTT assay, agarose gel electrophoresis and flow cytometry were used to evaluate HepG2 cells proliferation and apoptosis. The role of caspase activities were assayed using caspase apoptosis detection kit. Western blot analysis was used to evaluate the level of Bcl-2/Bax expression. Our results indicate that norcantharidin inhibited HepG2 cell growth in a time- and dose-dependent manner by MTT assay. HepG2 cells treated with norcantharidin showed typical characteristics of apoptosis including the DNA fragmentation. The activities of caspase-3, -9 were up-regulated after norcantharidin treatment. By western blot analysis, we found the level of Bcl-2 were down-regulated, whereas, the level of Bcl-2 Up-regulated.so we suggest that up-regulation of mitochondrial Bax expression and down-regulation of Bcl-2 expression participated in the apoptosis induced by NCTD. These results suggest that norcantharidin triggers apoptosis in hepato cancer cell lines via the activation of the caspses, mitochondrial pathways, and that this agent may be useful for developing new therapeutic regimens for the treatment of colorectal carcinoma.






Similar content being viewed by others
References
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132(7):2557–2576
Carrel JE, Eisner T (1974) Cantharidin: potent feeding deterrent to insects. Science 183:755–757
Wang GS (1989) Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol 26:147–162
Peng F, Wei YQ, Tien L et al (2002) Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol 128:223–230
Chen YN, Chen JC, Yin SC et al (2002) Effector mechanisms of norcantharidin induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer 100:158–165
Sun ZX, Ma QW, Zhao TD et al (2000) Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol 6:263–265
Hong CY, Huang SC, Lin SK et al (2000) Norcantharidin-induced post-G(2)/M apoptosis is dependent on wild-type p53 gene. Biochem Biophys Res Commun 276:278–285
Yi SN, Li MF, Xu YH (1989) Effects of sodium norcantharidate on granulopoiesis in normal and irradiated mice. Bull Hunan Med Univ 14:122–124
Yi SN, Luo FY, Sun JQ (1988) Preliminary study on the mechanism of increasing leukocyte count induced by sodium norcantharidate. Bull Hunan Med Coll 13:327–330
Wang GS (1989) Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol 26:147–162
Herrmann M, Lornz HM, Voll R et al (1994) A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 22:5506–5507
Kok S-H, Cheng S-J, Hong C-Y et al (2005) Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett 217:43–52
Yang EB, Tang WY, Zhang K et al (1997) Norcantharidin inhibits growth of human HepG2 cell transplanted tumor in nude mice and prolongs host survival. Cancer Lett 117:93–98
Hong CY, Huang SC, Lin SK et al (2000) Norcantharidin-induced post-G2/M apoptosis is dependent on wild-type p53 gene. Biochem Biophys Res Commun 276:278–285
Chen YN, Chen JC, Yin SC et al (2002) Effector mechanisms of norcantharidin induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer 100:158–165
Peng F, Wei YQ, Tian L et al (2002) Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. Cancer Res Clin Oncol 4:223–230
Eastman A, Rigas JR (1999) Modulation of apoptosis signaling pathways and cell cycle regulation. Semin Oncol 26(Suppl 16):7–16
Barry MA, Reynolds JE, Eastman A (1993) Etoposide-induced apoptosis in human HL-60 cells is associated with intracellular acidification. Cancer Res 53:2349–2357
Gorczyca W, Gong J, Ardelt B et al (1993) The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res 53:3186–3192
Wei YQ, Zhao X, Kariya Y et al (1994) Induction of apoptosis by quercetin: involvement of heat shock protein. Cancer Res 54:4952–4957
Kawazoe N, Watabe M, Masuda Y et al (1999) Tiami1 is involved in the regulation of bufalin-induced apoptosis in human leukemia cells. Oncongene 18:2413–2421
Hill PA, Tumber A, Meikle MC (1997) Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology 138:3849–3858
Mizukami S, Kikuchi K, Higuchi T et al (1999) Imagine of caspase-3 activation in HeLa cells stimulated with etoposide using a novel fluorescent probe. FEBS Lett 453:356–360
Nagata S (2000) Apoptotic DNA fragmentation. Exp Cell Res 10:12–18
Villa P, Kaufmann SH, Eamshaw WC (1997) Caspase and caspase inhibitors. Trends Biochem Sci 22:388–393
Stennicke HR, Salvesen S (1998) Properties of the caspases. Biochim Biophys Aeta 1387:1731
Salvesen GS, Dixit VM (1997) Caspase: intracellular signaling by proteolysis. Cell 91:443–446
Takahashi A, Eamshaw WC (1996) ICE-related proteases in apoptosis. Curr Opin Gene Dev 6:50–55
Enari M, Sakahira H, Yokoyama H et al (1998) A caspase-activated Dnase that degrades DNA during apoptosis and inhibitor ICAD. Nature 391:43–50
Kroemer G (1997) The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med 3:614–619
Sharpe JC, Arnoult D, Youle RJ (2004) Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta 1644(2–3):107–113
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1311
Grooss A, MeDonnell JM, Korsmeyer SJ (1999) Bcl-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Joza N (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410(6828):549–554
Harris MH, Thompson CB (2000) The role of the Bel-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ 7(12):1182–1191
Shi Y (2001) A structural view of mitochondria-mediated apoptosis. Nat Struct Biol 8(5):394–401
Acknowledgments
We thank Shi-quan Liu, Department of Oncology, Zhongnan Hospital of Wuhan University, for providing the HepG2 cell line and the technical support of cell culture, and Jun Luo, Department of Pathology, for exceptional technical assistance in flow cytometry analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11033-012-1620-1
An erratum to this article can be found at http://dx.doi.org/10.1007/s11033-012-2336-y
Rights and permissions
About this article
Cite this article
Chang, C., Zhu, Y., Tang, X. et al. The anti-proliferative effects of norcantharidin on human HepG2 cells in cell culture. Mol Biol Rep 38, 163–169 (2011). https://doi.org/10.1007/s11033-010-0090-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0090-6