Abstract
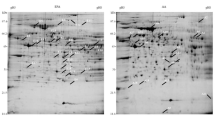
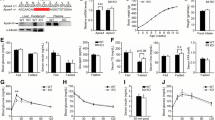
To investigate the effect of apolipoprotein E (apoE) on cytokine expression profile of the liver of young mice, quantitative RT-PCR (qRT-PCR) assay and cytokine antibody array for multiplex analysis of 62 cytokines have been used to analyze characteristics of expression of cytokines in the liver of 6-week-old apoE-null (apoE−/−) mice. The levels of plasma cytokines were also analyzed. The mRNA level of IL-1β, IL-2, IL-6, ICAM-1, VCAM-1, MCP-1, NF-κB (p65), IFN-γ and IκB-α were increased significantly in apoE−/− mice comparative to wild-type (WT) mice. IL-4, IL-10 and GM-CSF, however, were slightly decreased. Compared with WT, levels of 21 cytokines altered twofold or more in apoE−/− mice, including 10 cytokines increased and 11 decreased. Expression patterns of IL-1β, IL-2, IL-4, IL-6, IL-10, GM-CSF, IFN-γ and VCAM-1 showed identical trend between cytokine antibody array and qRT-PCR analysis. Moreover, levels of IL-1β, IFN-γ and IL-6 in the plasma were elevated, while IL-4 was lightly decreased in apoE−/− mice compared to those in WT mice. These results implied that promotion of type I immune response in the liver of young apoE−/− mice due to alteration of these cytokines, and the phenotypes may be caused by the regulation of NF-κB. The inflammation and lipid metabolism dysfunction in the liver cooperated in dysfunction of the liver in young apoE−/− mice.


Similar content being viewed by others
References
Pepe MG, Curtiss LK (1986) Apolipoprotein E is a biologically active constituent of the normal immunoregulatory lipoprotein, LDL-In. J Immunol 136:3716–3723
Kelly ME, Clay MA, Mistry MJ, Hsieh-Li HM, Harmony JA (1994) Apolipoprotein E inhibition of proliferation of mitogen-activated T lymphocytes: production of interleukin 2 with reduced biological activity. Cell Immunol 159:124–139. doi:10.1006/cimm.1994.1302
Roselaar SE, Daugherty A (1998) Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res 39:1740–1743
de Bont N, Netea MG, Demacker PN, Kullberg BJ, van der Meer JW, Stalenhoef AF (2000) Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae. Eur J Clin Invest 30:818–822. doi:10.1046/j.1365-2362.2000.00715.x
Ali K, Middleton M, Pure E, Rader DJ (2005) Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ Res 97:922–927. doi:10.1161/01.RES.0000187467.67684.43
Xiao N, Yin M, Zhang L, Qu X, Du H, Sun X, Mao L, Ren G, Zhang C, Geng Y, An L, Pan J (2009) Tumor necrosis factor-alpha deficiency retards early fatty-streak lesion by influencing the expression of inflammatory factors in apoE-null mice. Mol Genet Metab 96:239–244. doi:10.1016/j.ymgme.2008.11.166
Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR (2007) Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation 115:2049–2054. doi:10.1161/CIRCULATIONAHA.106.665570
Ditiatkovski M, Toh BH, Bobik A (2006) GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arterioscl Throm Vas 26:2337–2344. doi:10.1161/01.ATV.0000238357.60338.90
Ye HY, Yin M, Shang YJ, Dai XD, Zhang SQ, Jing W, Du HQ, Zhang L, Pan J (2008) Differential expressions of lipid metabolism related genes in the liver of young apoE knockout mice. Sheng Li Xue Bao 60:51–58
Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS, Reddy JK (1998) Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem 273:15639–15645. doi:10.1074/jbc.273.25.15639
Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45:1169–1196. doi:10.1194/jlr.R300019-JLR200
Delerive P, Fruchart JC, Staels B (2001) Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol 169:453–459. doi:10.1677/joe.0.1690453
Barbier O, Torra IP, Duguay Y, Blanquart C, Fruchart JC, Glineur C, Staels B (2002) Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscl Throm Vas 22:717–726. doi:10.1161/01.ATV.0000015598.86369.04
Youssef J, Badr M (2004) Role of peroxisome proliferator-activated receptors in inflammation control. J Biomed Biotechnol 2004:156–166. doi:10.1155/S1110724304308065
Lehrke M, Millington SC, Lefterova M, Cumaranatunge RG, Szapary P, Wilensky R, Rader DJ, Lazar MA, Reilly MP (2007) CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J Am Coll Cardiol 49:442–449. doi:10.1016/j.jacc.2006.09.034
Matloubian M, David A, Engel S, Ryan JE, Cyster JG (2000) A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol 1:298–304. doi:10.1038/79738
Fan J, Heller NM, Gorospe M, Atasoy U, Stellato C (2005) The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. Eur Respir J 26:933–947. doi:10.1183/09031936.05.00120204
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundations of China (30571024) and Science and Technology Projects of Shandong (J06L11) to J. Pan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, M., Zhang, L., Sun, Xm. et al. Lack of apoE causes alteration of cytokines expression in young mice liver. Mol Biol Rep 37, 2049–2054 (2010). https://doi.org/10.1007/s11033-009-9660-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9660-x